Details of DPV and References
DPV NO: 16 June 1970
Family: Secoviridae
Genus: Nepovirus
Species: Arabis mosaic virus | Acronym: ArMV
Arabis mosaic virus
A. F. Murant Scottish Horticultural Research Institute, Invergowrie, Dundee, Scotland
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by Smith & Markham (1944).
- Selected synonyms
- Raspberry yellow dwarf virus (Rev. appl. Mycol. 37: 669; 39: 261)
- Rhabarber-Mosaik-Virus (rhubarb mosaic virus) (Rev. appl. Mycol. 37: 64; 39: 561)
- Rhabarber-Mosaik-Virus (rhubarb mosaic virus) (Rev. appl. Mycol. 37: 64; 39: 561)
- An RNA-containing virus which has isometric particles about 30 nm in diameter and occurs in Europe. It is readily sap-transmissible, has a wide host range, infects the seed of many host plants, and is transmitted by the nematode Xiphinema diversicaudatum.
Main Diseases
Causes yellow dwarf of raspberry, mosaic and yellow crinkle of strawberry, stunt mottle of cucumber, chlorotic stunt of lettuce, stunting and necrosis of celery, and yellow net of Forsythia intermedia, and is one of the causes of mosaic of rhubarb. It occurs in many other plants including sugar beet, hop, horseradish, Narcissus, rose, Sambucus nigra, Ligustrum vulgare, white clover and grapevine. Grapevine plants more commonly contain grapevine fanleaf virus, which is distantly serologically related to arabis mosaic virus, and is the subject of another Description. In association with viruses of the prunus necrotic ringspot type it causes one form of rasp-leaf of cherry.
Geographical Distribution
Natural spread is not reported outside Europe. The virus has been found in stocks of rhubarb in Canada (R. Stace-Smith, personal communication).
Host Range and Symptomatology
Occurs naturally in many species of wild and cultivated monocotyledonous and dicotyledonous plants. It infected 93 species in 28 dicotyledonous families when transmitted by mechanical inoculation (Schmelzer, 1962), and it is also reported to infect the roots of the gymnosperm Chamaecyparis lawsoniana. It infects almost all commonly used herbaceous test plants, but isolates of the virus differ in virulence.
- Diagnostic species
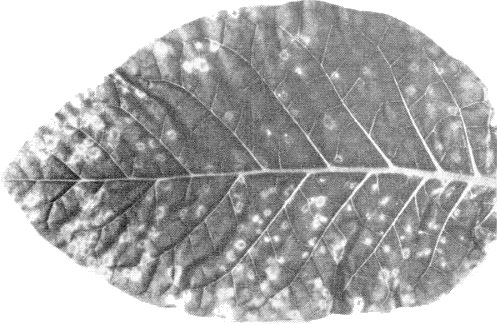
- Chenopodium amaranticolor and C. quinoa. Chlorotic local lesions (Fig. 1);
systemic chlorotic mottle (Fig. 5).
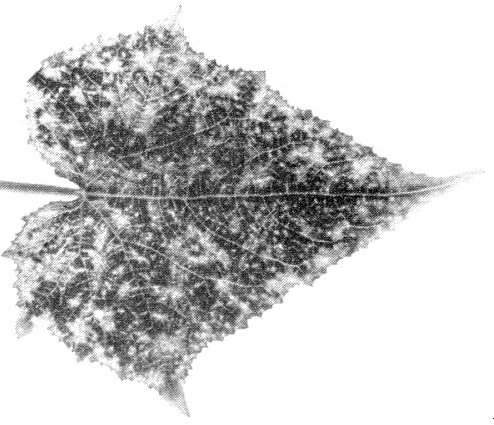
- Cucumis sativus (cucumber). Chlorotic local lesions; systemic yellow spots or veinbanding (Fig. 3), subsequently fading. Plants then stop growing.
- Nicotiana tabacum cv. White Burley (tobacco). Chlorotic or necrotic local lesions. Some isolates give systemic yellow spots and rings (Fig. 2) and line patterns. Leaves produced later appear almost normal but contain virus.
- Phaseolus vulgaris cv. The Prince (French bean). Faint chlorotic local lesions; systemic necrosis and distortion.
- Petunia hybrida. Local chlorotic lesions or necrotic rings. Systemic vein-clearing or chlorotic rings and line-patterns (Fig. 4). Leaves produced subsequently are symptomless but contain virus.
- Cucumis sativus (cucumber). Chlorotic local lesions; systemic yellow spots or veinbanding (Fig. 3), subsequently fading. Plants then stop growing.
- Propagation species
- Petunia hybrida is a useful plant for maintaining cultures; P. hybrida and
Nicotiana clevelandii are good sources of virus for purification.
- Assay species
- Chenopodium amaranticolor can be used to assay virulent isolates; with avirulent isolates, lesions on most hosts are too indistinct to count. Cucumis sativus is a convenient ‘bait plant’ in nematode transmission tests.
Strains
Most isolates seem to differ little from the type strain, apart from minor differences in virulence. However, the strain from hop (Bock, 1966) infects only Chenopodium amaranticolor and C. quinoa of the herbaceous test plants tried, and differs serologically from the type strain; its vector is unknown.
Transmission by Vectors
The type strain is transmitted by the free-living, soil-inhabiting nematodes, Xiphinema diversicaudatum (Jha & Posnette, 1959; Harrison & Cadman, 1959) and X. coxi (Fritzsche & Schmidt, 1963). Larvae and adults of X. diversicaudatum both transmit, but the adult does not pass the virus to its progeny nor is the virus retained after moulting. X. diversicaudatum can acquire the virus after access to infected plants for 1 day and can inoculate bait plants in 3 days (Jha & Posnette, 1961). The nematodes retain virus for at least 31 days when kept in fallow soil (Jha & Posnette, 1961) and for at least 8 months when kept on a virus-immune variety of raspberry (Harrison & Winslow, 1961).
Virus-like particles were associated with the cuticular linings of the lumina of the odontophore (stylet extension) and the oesophagus of X. diversicaudatum which had fed on plants infected with arabis mosaic virus (Taylor & Robertson, 1970).
Transmission through Seed
Seed-borne in at least 15 spp. in 12 plant families. In many hosts more than 10%, and in some nearly 100%, of progeny seedlings are infected. Many plants infected through seed show no symptoms (Lister & Murant, 1967; Murant & Lister, 1967).
Transmission by Dodder
Cuscuta subinclusa and C. californica transmitted the virus frequently, C. campestris only occasionally (Schmelzer, 1962).
Serology
Antisera with titres of 1/500-1/1000 are readily obtained. Precipitin tests in tubes, microprecipitin tests, or gel-diffusion tests in 1% agar are satisfactory. The virus produces one line of precipitation in gel-diffusion tests.
Relationships
Most isolates seem closely related serologically to the type strain (Cadman, 1960; Hollings, 1963). The hop line-pattern strain can be distinguished from the type strain by spur-formation in gel-diffusion tests (Bock, 1966), but the extent of difference was not reported.
Harrison (1958) found that cross-protection occurred between different isolates but Jha (1961) and Hollings (1963) reported that protection was sometimes incomplete or non-reciprocal. Schmelzer (1963) found that cross-protection was more consistent between isolates of arabis mosaic virus than between those of tomato black ring virus.
Arabis mosaic virus is distantly serologically related to grapevine fanleaf virus (Cadman, Dias & Harrison, 1960). It also resembles in many properties other nepoviruses (strawberry latent ringspot, tobacco ringspot, tomato black ring and tomato ringspot) but is unrelated to them serologically.
Stability in Sap
In Petunia hybrida sap, the virus lost infectivity after 10 min at 55-61°C, storage at room temperature for 1-2 weeks, or dilution to 10-3-10-5 (Harrison, 1958). Some strains survive longer periods in sap at room temperature (Hollings, 1963; Schmelzer, 1962).
Purification
A modification of Steere’s butanol/chloroform method is useful (Harrison & Nixon, 1960).
Properties of Particles
The particles are all the same size but sediment as three components, apparently empty protein shells (T) and two kinds of nucleoprotein (M and B).
- Sedimentation coefficients (s20,w) (svedbergs): 53 (T),
93 (M), 126 (B) (R. Stace-Smith, personal communication).
- Other physical properties unknown.
Particle Structure
Isometric, about 30 nm in diameter with a 5 or 6-sided angular outline (Harrison & Nixon, 1960). The protein shell may be composed of 42 morphological subunits (Agrawal, 1967). Electron micrographs show some particles completely, some partially and some not penetrated by negative stain (Fig. 6). These particles possibly correspond to the T, M and B components.
Particle Composition
RNA: Single-stranded; about 27% (M) and 41% (B) of the particle weight (estimated from the sedimentation coefficients).
Relations with Cells and Tissues
In Chenopodium amaranticolor, inclusion bodies form adjacent to the cell nuclei; some of them contain particles arranged in spheroidal shells (Gerola et al., 1965; I. M. Roberts, personal communication).
Notes
Diseases caused by this virus are distributed patchily in crops because of the slow migration of the soil-inhabiting vectors, Xiphinema spp. The virus tends to occur in soils together with strawberry latent ringspot virus, with which it shares the vector, X. diversicaudatum. These two viruses are difficult to distinguish from each other with certainty by their reactions in host plants but are serologically unrelated. As with other nepoviruses, serological tests provide the only reliable means of identification.
Figures
References list for DPV: Arabis mosaic virus (16)
- Agrawal, J. Ultrastruct. Res. 17: 84, 1967.
- Bock, Ann. appl. Biol. 57: 131, 1966.
- Cadman, Virology 11: 653, 1960.
- Cadman, Dias & Harrison, Nature, Lond. 187: 577, 1960.
- Fritzsche & Schmidt, Naturwissenschaften 50: 163, 1963.
- Gerola, Bassi & Betto, Caryologia 18: 353, 1965.
- Harrison, Ann. appl. Biol. 46: 221, 1958.
- Harrison & Cadman, Nature, Lond. 184: 1624, 1959.
- Harrison & Nixon, Virology 12: 104, 1960.
- Harrison & Winslow, Ann. appl. Biol. 49: 621, 1961.
- Hollings, J. hort. Sci. 38: 138, 1963.
- Jha, J. hort. Sci. 36: 219, 1961.
- Jha & Posnette, Nature, Lond. 184: 962, 1959.
- Jha & Posnette, Virology 13: 119, 1961.
- Lister & Murant, Ann. appl. Biol. 59: 49, 1967.
- Murant & Lister, Ann. appl. Biol. 59: 63, 1967.
- Schmelzer, Phytopath. Z. 46: 105, 1962.
- Schmelzer, Phytopath. Z. 46: 315, 1963.
- Smith & Markham, Phytopathology 34: 324, 1944.
- Taylor & Robertson, Rep. Scott. hort. Res. Inst., 1969: 63, 1970.