Details of DPV and References
DPV NO: 28 October 1970
Family: Secoviridae
Genus: Nepovirus
Species: Grapevine fanleaf virus | Acronym: GFLV
There is a more recent description of this virus: DPV 385
Grapevine fanleaf virus
W. B. Hewitt University of California, San Joaquin Valley Agricultural Research and Extension Center, Parlier, California, USA
G. Martelli Institute of Plant Pathology, University of Bari, Italy
H. F. Dias Canada Agriculture Research Station, Vineland, Ontario, Canada
R. H. Taylor Victorian Plant Research Institute, Burnley, Melbourne, Australia
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by Hewitt (1950b), Cadman, Dias & Harrison (1960),
Dias (1963), Martelli & Hewitt (1963a), Vuittenez (1963), and Taylor
& Hewitt (1964).
- Selected synonyms
- Grapevine arriciamento virus (Rev. appl. Mycol. 9: 83)
- Grapevine court-noué virus (Rev. appl. Mycol. 9: 83)
- Grapevine infectious degeneration virus (Rev. appl. Mycol. 28: 2)
- Grapevine Reisigkrankheit Virus (Rev. appl. Mycol. 13: 213)
- Grapevine roncet virus (Rev. appl. Mycol. 9: 83)
- Grapevine urticado virus (Dias, 1950a)
- Others: (Rev. appl. Mycol. 11: 280; 28: 514; 38: 617; 42: 513; 43: 2484)
- Grapevine court-noué virus (Rev. appl. Mycol. 9: 83)
- A virus with isometric particles about 30 nm in diameter, transmitted by nematodes (Xiphinema spp.), commonly to Vitis species and rarely to other species, and readily transmitted by manual inoculation to a moderate range of other plants. Common in many regions where grapevine (V. vinifera) has been grown for long periods, and where hybrid rootstocks are used for grapevine culture.
Main Diseases
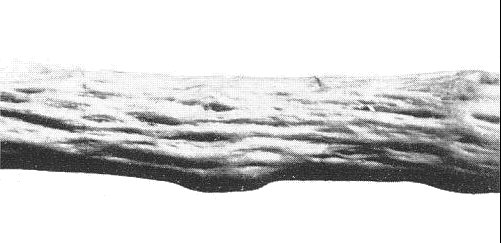
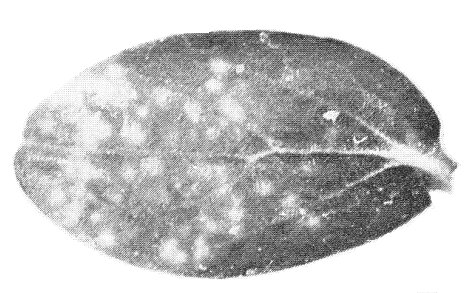
Specific strains cause fanleaf (Fig. 1 & Fig. 3), yellow mosaic (Fig. 4), and veinbanding diseases (Fig. 2) of grapevine (V. vinifera). Some isolates are associated with leaf enation, bark- and wood-pitting, and flat trunk diseases of grapevine.
Geographical Distribution
Occurs in almost all temperate regions of the world wherever Vitis vinifera and hybrid rootstocks of grapevines are grown. The virus is apparently native to V. vinifera and originated in the same area as the host grapevine (Hewitt, 1968).
Host Range and Symptomatology
Restricted in nature to Vitis spp., perhaps because of vector specificity and the limited host ranges of the vectors. It may be transmitted experimentally to numerous herbaceous species. (Baldacci et al., 1962; Brückbauer & Rüdel, 1961b; Cadman et al., 1960; Dias, 1963; Martelli & Hewitt, 1963a; Taylor & Hewitt, 1964; Vuittenez, 1963).
- Diagnostic species
- The virus in grapevine may be diagnosed either by grafting to Vitis
spp. or by inoculating it manually to herbaceous plants and identifying it
serologically.
- Symptoms in V. vinifera, V. rupestris, many other Vitis spp. and interspecific hybrids are green or yellow systemic mosaic, rings, line patterns (Fig. 3), and flecks; leaf deformities include open marginal and petiolar sinuses, and prominent marginal teeth; cane deformities include uneven internode spacing, double nodes, flat canes, and fasciations (Fig.1), and cells of phloem and xylem tissues have trabeculae (Gifford et al., 1956).
- Chenopodium amaranticolor and C. quinoa. Chlorotic local lesions may develop in plants grown in shade at about 18°C; systemically infected leaves become mottled (Fig. 6) with vein clearing, and may be deformed or necrotic. Symptoms fade as the plants age.
- Cucumis sativus (cucumber). May show chlorotic local lesions (Fig. 7). Systemically infected leaves develop chlorotic or necrotic mosaic, mottle, blotches, vein flecks or rings. Some strains do not infect.
- Gomphrena globosa. Local lesions (chlorotic spots becoming reddish) may develop; systemically infected leaves show chlorotic areas, and are twisted.
- Phaseolus vulgaris cv. Bountiful (bean). Chlorotic local lesions may form. Systemically infected leaves show mottle, vein clearing, line patterns, chlorotic blotches, and distortion.
- Symptoms in V. vinifera, V. rupestris, many other Vitis spp. and interspecific hybrids are green or yellow systemic mosaic, rings, line patterns (Fig. 3), and flecks; leaf deformities include open marginal and petiolar sinuses, and prominent marginal teeth; cane deformities include uneven internode spacing, double nodes, flat canes, and fasciations (Fig.1), and cells of phloem and xylem tissues have trabeculae (Gifford et al., 1956).
- Propagation species
- Chenopodium amaranticolor, C. quinoa, Cucumis sativus, Gomphrena globosa
and Phaseolus vulgaris are sources of virus for purification. G.
globosa is good for maintaining cultures.
- Assay species
- No good local lesion host has been reported, but virus may be assayed by systemic symptoms in above species. Serology must be used to identify fanleaf virus. Seedlings and rooted cuttings of V. vinifera and V. rupestris are good bait plants in studies with nematode vectors.
Strains
Many variants have been distinguished by differences in host range and symptomatology in grapevine. The best-known strains, often mixed in field-grown grapevines, are:
Fanleaf strain of Hewitt (1950b). On new spring growth, varying degrees of mottle, leaves deformed, open petiolar and marginal sinuses, prominent leaf vein tips, excessive growth of lateral shoots, canes deformed, with irregular internodes and double nodes (Fig. 1 & Fig. 3).
Yellow mosaic strain of Hewitt (1950a) and Dias (1950b). Leaves, shoots, and flower forms of spring growth are often yellow or greenish yellow (Fig. 4). Leaves show various yellow mosaic patterns but seldom have severe deformities. Also known as grapevine clorose infecciosa virus (Dias, 1950b) and grapevine panachure virus (Rev. appl. Mycol. 31: 590).
Veinbanding strain of Goheen & Hewitt (1962), and Martelli & Hewitt (1963a). Prominent yellow and greenish-yellow bands, usually forming in older leaves after mid-season and in many varied leaves thereafter, along veins of the first, second and third order (Fig. 2).
Other strains. Strains of fanleaf virus are associated with the wood pitting disease (Graniti, 1964; Graniti & Martelli, 1965) (Fig. 5) and also the enation disease (Martelli et al., 1966).
Transmission by Vectors
Transmitted by the nematodes Xiphinema index (Hewitt, Raski & Goheen, 1958) and X. italiae (Cohn, Tanne & Nitzany, 1970). All larval stages transmit but lose virus after moulting. Adults transmit and can retain virus for several months even when held in the root zone of a virus-immune host (Raski et al., 1965). Virus may be acquired or transmitted within a few minutes of feeding (Das & Raski, 1968). Vectors do not transmit virus to their progeny.
Transmission through Seed
Rarely if ever transmitted to embryo of Vitis vinifera; virus is abundant in endosperm of seed from infected vines but not in embryo. It is transmitted through seed to seedling, however, in Chenopodium amaranticolor (Dias, 1963), C. quinoa (Brückbauer & Rüdel, 1961a), and soybean (Cory & Hewitt, 1968). Virus occurs in pollen of grapevine and herbaceous hosts (Cory & Hewitt, 1968).
Transmission by Dodder
Cuscuta campestris did not transmit fanleaf virus to Chenopodium amaranticolor (Dias, 1963).
Serology
The virus is moderately immunogenic and is easily prepared free of host proteins. In gel-diffusion tests it reacts with antisera to produce a distinct single band of precipitate.
Relationships
Different strains of fanleaf virus have most of their antigenic groups in common (Cadman et al., 1960; Barabino, 1963; Dias & Harrison, 1963; Martelli & Hewitt, 1963b; Taylor & Hewitt, 1964), and are all distantly serologically related to arabis mosaic virus.
In plant-protection tests, avirulent isolates protect against virulent isolates either completely or partially (Dias & Harrison, 1963; Taylor & Hewitt, 1964).
Stability in Sap
In sap of herbaceous hosts the thermal end point (10 min) is 60-65°C and the dilution end point 10-3-10-4. The virus retains infectivity for 15-30 days at about 20°C (Brückbauer & Rüdel, 1961b); Cadman et al., 1960; Dias, 1963; Taylor & Hewitt, 1964). The virus is precipitated by 40% acetone, 40% ethanol or a 30-35% saturated solution of ammonium sulphate (Dias, 1963).
Purification
The n-butanol method of Tomlinson, Shepherd & Walker or the butanol-chloroform method of Steere, followed by density gradient centrifugation, gives preparations free of plant protein (Dias & Harrison, 1963; Martelli & Hewitt, 1963b; Taylor & Hewitt, 1964). Yield of pure virus is often low; young Phaseolus vulgaris plants grown at 15-18°C in shade give 1 mg virus/200 g tissue; G. globosa gives 1 mg virus/400 g tissue.
Properties of Particles
Little information available. Isoelectric point is about pH 4; the virus is precipitated at this point.
Particle Structure
Particles are isometric with angular outlines c. 30 nm in diameter (Fig. 8) (Dias & Harrison, 1963; Martelli & Hewitt, 1963b); some particles appear hollow (Dias, 1963) when stained in 2% sodium phosphotungstate at pH 7; stain penetrates some particles and they contrast poorly.
Particle Composition
Unknown.
Relations with Cells and Tissues
Particles of the same size and shape as those of the virus have been found in rows in the lumens of parenchyma cells of grapevine roots and mesophyll cells of C. amaranticolor leaves (Gerola, Bassi & Belli, 1969).
Notes
Grapevine fanleaf virus may be transmitted mechanically from grapevine tissue to herbaceous plants by grinding 1 g young leaf tissue in 5 ml 2.5% nicotine solution (Cadman et al., 1960), or in phosphate buffer at pH 8. It is also transmissible in sap from root tips or etiolated shoots of diseased grapevine ground in phosphate buffer at pH 7. The virus is readily transmissible in sap from herbaceous hosts, or in partially purified or purified form to etiolated leaves of grapevine (Hewitt & Cory, 1965).
Figures
References list for DPV: Grapevine fanleaf virus (28)
- Baldacci, Belli, Betto & Refatti, Annali Fac. Agr. Univ. Milano 10: 23, 1962.
- Barabino, Hort. Res. 3: 27, 1963.
- Brückbauer & Rüdel, Wein-Wiss. 16: 177, 1961a.
- Brückbauer & Rüdel, Wein-Wiss. 16: 197, 196lb.
- Cadman, Dias & Harrison, Nature, Lond. 187: 577, 1960.
- Cohn, Tanne & Nitzany, Phytopathology 60: 181, 1970.
- Cory & Hewitt, Phytopathology 58: 1316, 1968.
- Das & Raski, Nematologica 14: 55, 1968.
- Dias, Comunic. ao 13e Congr. Luso-Esp. Prog. Cienc. 5: 167, 1950a.
- Dias, Comunic. ao 13e Congr. Luso-Esp. Prog. Cienc. 5: 177, 1950b;
- Dias, Ann. appl. Biol. 51: 85, 1963.
- Dias & Harrison, Ann. appl. Biol. 51: 97, 1963.
- Gerola, Bassi & Belli, G. bot. ital. 103: 271, 1969.
- Gifford, Hewitt, Graham & Lamoureux, Bull. Calif. Dep. Agric. 45: 268, 1956.
- Goheen & Hewitt, Am. J. Enol. Vitic. 13: 73, 1962.
- Graniti, Phytopath. Mediterranea. 3: 19, 1964.
- Graniti & Martelli, Proc. Int. Conf. on Virus Vector Peren. Hosts, Univ. Cal. Agr. Sci., Davis: 168, 1965.
- Hewitt, Bull. Calif. Dep. Agric. 39: 61, 1950a.
- Hewitt, Bull. Calif. Dep. Agric. 39: 62, 1950b.
- Hewitt, Rev. appl. Mycol. 47: 433, 1968.
- Hewitt & Cory, Proc. Int. Conf. Virus Vector Peren. Hosts, Univ. Cal. Agr. Sci., Davis: 322, 1965.
- Hewitt, Raski & Goheen, Phytopathology 48: 586, 1958.
- Martelli, Annali Fac. Agr. Univ. Bari 16: 307, 1962.
- Martelli, Graniti, Lamberti & Quacquarelli, Phytopath. Mediterranea 5: 122, 1966.
- Martelli & Hewitt, Phytopath. Mediterranea 2: 275, 1963a.
- Martelli & Hewitt, Phytopath. Mediterranea 2: 285, 1963b.
- Raski, Hewitt, Goheen, Taylor & Taylor, Nematologica 11: 349, 1965.
- Taylor & Hewitt, Aust. J. agric. Res. 15: 571, 1964.
- Vuittenez, C. r. hebd. Séanc. Acad. Agric. Fr. 49: 795, 1963.