Details of DPV and References
DPV NO: 39 October 1970
Family: Bunyaviridae
Genus: Tospovirus
Species: Tomato spotted wilt virus | Acronym: TSWV
Tomato spotted wilt virus
T. S. Ie Laboratorium voor Virologie, Binnenhaven, Wageningen, The Netherlands
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by Samuel, Bald & Pittman (1930),
Klinkowski & Uschdraweit (1952),
reviewed by Best (1968).
Selected synonyms
- Kromnek virus (Rev. appl. Mycol. 13: 124)
- Lycopersicum virus 3 (Rev. appl. Mycol. 36: 303)
- Pineapple yellow spot virus (Rev. appl. Mycol. 11: 116; 11: 586; 19: 483)
- Tomato bronze leaf virus (Rev. appl. Mycol. 30: 450)
- ‘Vira cabeça’ virus (Rev. appl. Mycol. 20: 501; 45: 756)
- Lycopersicum virus 3 (Rev. appl. Mycol. 36: 303)
-
An RNA-containing virus with membrane-bound isometric particles 70-90 nm in diameter. It is transmitted by thrips and by inoculation of sap, has a very wide host range and is common in temperate and subtropical regions throughout the world.
Main Diseases
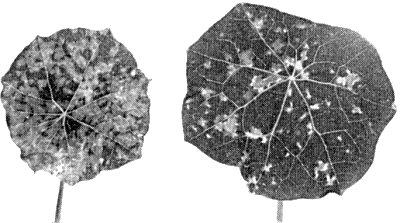
Causes a range of chlorotic, necrotic, stunting and enation symptoms in all parts of its many hosts. The bronzing symptoms in tomato leaves (Fig. 1, Fig. 6) and the one-sided growth (kromnek) of tobacco and tomato are characteristic. Leaf enations occur in Dahlia, flower deformations and colour breaking of the petals in Dahlia and Zinnia. The great variability in symptom expression is probably largely due to the complex of mixed strains.
Geographical Distribution
Common in temperate and subtropical regions throughout the world.
Host Range and Symptomatology
Host range is very wide; found in 166 plant species in 34 families, including 7 monocotyledonous families (Klinkowski & Uschdraweit, 1952; Smith, 1957; Best, 1968). Easily transmissible by inoculation of sap using abrasives, especially when extracts are made in neutral buffer, containing reducing agents.
-
Diagnostic species
- Petunia hybrida
cvs. Pink Beauty and Minstrel. Local necrotic lesions 2-4 days after inoculation; not systemic (Fig. 2). -
Nicotiana tabacum cv. Samsun NN (tobacco), N. clevelandii and
N. glutinosa. Local necrotic lesions, followed by systemic necrotic
patterns and leaf deformation.
- Cucumis sativus (cucumber). Cotyledons develop local chlorotic spots with necrotic centres, 4-5 days after inoculation (Fig. 3).
- Vinca rosea. Local black spots, 10-14 days after inoculation, leaves sometimes yellowing and abscissing; systemic mosaic and deformation.
- Tropaeolum majus. Inoculated leaves symptomless; after 8-12 days a systemic mosaic pattern of yellow and dark green specks develops (Fig. 5), sometimes also with necrotic spots.
- Cucumis sativus (cucumber). Cotyledons develop local chlorotic spots with necrotic centres, 4-5 days after inoculation (Fig. 3).
-
Propagation species
- Tropaeolum majus
and Gomphrena globosa are suitable plants for maintaining cultures. Leaves with symptoms contain much virus and are good inoculum sources. Good sources of virus for purification are the systemically infected leaves of Nicotiana rustica and N. glutinosa.Assay species
- Petunia hybrida
(cvs. Pink Beauty and Minstrel) is the best local lesion host. Local necrotic lesions 2-3 days after inoculating plants in the glasshouse, but also in detached leaves in Petri dishes under artificial illumination (Selman & Milne, 1961).
Strains
Many minor variants, giving symptoms differing in severity, have been isolated. The most stable and important of these are: strains TB (tipblight), N (necrotic), R (ringspot), M (mild), VM (very mild) of Norris (1946); strains A, B, C1, C2, D, and E of Best (1968); the ‘vira-cabeça’ strain (Fawcett, 1940; Kitajima, 1965); and the tomato tip blight strain (McWhorter & Milbrath, 1935, 1938).
Transmission by Vectors
Transmitted by the thrips Thrips tabaci, Frankliniella schultzei, F. occidentalis and F. fusca (Sakimura, 1961, 1962; Best, 1968). The virus is acquired by the larvae but not by the adults, whereas only adults transmit. Thus transmission is only by adults that fed on infected plants in the larval stage (Bald & Samuel, 1931). Shortest reported acquisition period is 15 min for T. tabaci. Latent (incubation) period is 4-10 days, depending on the vector species. Vectors are maximally infective 22-30 days after acquisition but sometimes retain the virus for life. They do not transmit virus to their progeny.
Transmission through Seed
Reported (96%) in Cineraria and tomato (Jones, 1944) but Crowley (1957) found only 1% infection; the virus is apparently carried in the testa, not in the embryo.
Transmission by Dodder
No reports.
Serology
Antisera of good titre are still not available. Best & Hariharasubramanian (1967) prepared an antiserum with a titre of 1/256 in precipitin tests. Feldman & Boninsegna (1968) obtained an antiserum with a titre of only 1/10 in gel-diffusion tests, using heated (70°C) infective sap as immunogen.
Stability in Sap
Physically and chemically one of the most unstable plant viruses. In sap, the thermal inactivation point (10 min) is 40-46°C, longevity in vitro at room temperature is 2-5 hr and dilution end point between 2 x 10-2 and 10-3. Infectivity rapidly falls at pH values below pH 5, and is maintained best at pH values near pH 7. It is greatly stabilized in plant extracts by adding reducing agents such as 0.01 M Na2SO3 or Na thioglycollate.
Purification
Because of its extreme instability in vitro, purification of tomato spotted wilt virus is still a problem.
1. Best (1968). Extract 100 g systemically infected Nicotiana glutinosa leaves in 500 ml ice-cold 0.01 M phosphate buffer (pH 7) containing Na2SO4 (0.07 M), Na2SO3 (0.01 M) and sodium diaminoethane-tetraacetate (Na-EDTA) (1 x 10-4 M). Concentrate and purify the virus by differential centrifugation and one or two cycles of sucrose density gradient centrifugation. Yields are about 1 mg virus per 100 g tissue.
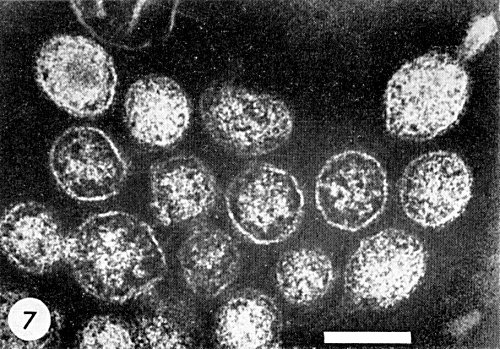
2. Black, Brakke & Vatter (1963). Extract 100 g systemically infected Nicotiana rustica leaves in 200 ml ice-cold 0.1 M potassium phosphate buffer pH 7, containing Na2SO3 (0.01 M). In this high ionic strength buffer, most of the infectivity is sedimented by low speed centrifugation (c. 1700 g for 30 min). Resuspend in 0.01 M Na2SO3 and concentrate by differential centrifugation. Sucrose density gradient centrifugation gives a visible zone with which most of the infectivity is associated. This method does not always give good results because it takes 8-10 hr and the virus is very unstable. Van Kammen, Henstra & Ie (1963) using nearly the same method as Black et al. confirm their experiences. The purified virus is not sufficiently free of cellular contaminants and the virus particles sometimes show a typical tail-like extrusion (Fig. 7).
Properties of Particles
Sedimentation coefficient (s20,w): 530 S (Best, 1968); 583 S (Black et al., 1963).
Particle Structure
Particles approximately isometric, c. 70-90 nm in diameter, apparently bounded by a membrane (Fig. 4, Fig. 7). The structure of the material inside the membrane is uncertain. The outer layer of the membrane seems to consist of a nearly continuous layer of projections c. 5 nm thick, which stain more densely than the membrane itself. Purified particles sometimes show a tail-like extrusion (Fig. 7).
Particle Composition
Best (1968) reported preliminary experiments which suggested that the particles contain about 20% lipid, 7% carbohydrate and 5% RNA, with the unusual base composition (molar percentage of nucleotides) of G38; A35; C9; U19. The proportions of the usual amino acids fall within the range found for other viruses except that the lysine and histidine content is high.
Relations with Cells and Tissues
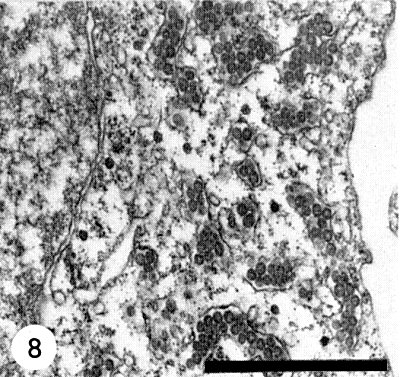
In the plant cell, the characteristic particles of tomato spotted wilt virus occur as clusters in cytoplasmic vacuoles, which are possibly cisternae of the endoplasmic reticulum (Fig. 8) (Ie, 1964; Milne & De Zoeten, 1967; Milne, 1970), and in the dilated lumen of the nuclear membrane (Kitajima, 1965). They have not so far been found in other cell organelles. The virus particles are found in the cells of roots, stems, leaves and petals. In the anthers of Tropaeolum majus they occur only in the endothecium tissue but never in the tapetum layer or in the pollen cells. In newly infected cells of young leaf tissue, typical dense masses occur in the cytoplasm between the ribosomes, sometimes in big complexes located near the nucleus. These dense masses are striated with a periodicity of about 5 nm (Ie, unpublished). The typical electron dense particles scattered singly between the ribosomes (Fig. 8), have no relationship at all with TSWV, but are normal constituents of the cells of some cultivars of Tropaeolum majus.
Acknowledgements
Photographs: Courtesy of Laboratory of Virology, State Agricultural University, Wageningen.
Figures

Systemically infected tomato leaves. Yellow specks and beginning of small necrotic spots, resulting in the typical ‘bronzing’ symptoms. Top leaves distorted.

Virus particles in an ultrathin section of a tomato leaf cell. Embedded in methacrylate, fixed with glutaraldehyde and OsO4, stained with uranyl acetate and lead citrate.

Systemically infected older tomato leaves with obvious yellow and necrotic spots. Distorted young fruit.
References list for DPV: Tomato spotted wilt virus (39)
- Bald & Samuel, Bull. Coun. scient. ind. Res., Melb. 54, 24 pp., 1931.
- Best, Adv. Virus Res. 13: 66, 1968.
- Best & Hariharasubramanian, Enzymologia 32: 128, 1967.
- Black, Brakke & Vatter, Virology 20: 120, 1963.
- Crowley, Aust. J. biol. Sci. 10: 449, 1957.
- Fawcett, Revta ind. agric. Tucumán 30: 221, 1940.
- Feldman & Boninsegna, Nature, Lond. 219: 183, 1968.
- Ie, Neth. J. Pl. Path. 70: 114, 1964.
- Jones, Phytopathology 34: 941, 1944.
- Kitajima, Virology 26: 89, 1965.
- Klinkowski & Uschdraweit, Phytopath. Z. 19: 269, 1952.
- McWhorter & Milbrath, Phytopathology 25: 897, 1935.
- McWhorter & Milbrath, Stn Circ. Ore. agric. Exp. Stn 128, 14pp., 1938.
- Milne, J. gen. Virol. 6: 267, 1970.
- Milne & De Zoeten, J. Ultrastruct. Res. 19: 398, 1967.
- Norris, Bull. Coun. scient. ind. Res., Melb. 202, 51 pp., 1946.
- Sakimura, Pl. Dis. Reptr 45: 766, 1961.
- Sakimura, in Biological transmission of disease agents, Ed. K. Maramorosch, New York, Academic Press, 1962.
- Samuel, Bald & Pittman, Bull. Coun. scient. ind. Res., Melb. 44, 64 pp., 1930.
- Selman & Milne, Pl. Path. 10: 100, 1961.
- Smith, Textbook of Plant Virus Diseases, 572 pp., London, Churchill, 1957.
- Van Kammen, Henstra & Ie, Virology 30: 574, 1966.