Details of DPV and References
DPV NO: 56 June 1971
Family: Alphaflexiviridae
Genus: Potexvirus
Species: Papaya mosaic virus | Acronym: PapMV
Papaya mosaic virus
D. E. Purcifull Department of Plant Pathology, Institute of Food & Agricultural Sciences, University of Florida, Gainesville, Florida, USA
E. Hiebert Department of Plant Pathology, Institute of Food & Agricultural Sciences, University of Florida, Gainesville, Florida, USA
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by
Conover (1962;
1964b) and
de Bokx (1965).
Synonym
- Papaya (papaw) mild mosaic virus (Rev. appl. Mycol. 41: 530)
An RNA-containing virus with filamentous particles c. 530 nm long. Infects several dicotyledonous plants and is readily sap-transmissible. Vector unknown. Causes a disease of minor importance in papaya in Florida, USA, and occurs in Venezuela.
Main Diseases
Causes leaf mosaic and stunting in papaya.
Geographical Distribution
Reported in USA (Conover, 1962; 1964b) and Venezuela (Cook & Zettler, 1970).
Host Range and Symptomatology
Papaya seems to be the only natural host, but 17 species in 9 dicotyledonous families have been infected experimentally. Readily sap-transmissible.
Diagnostic species
- Carica papaya
(papaya). Young seedlings in the greenhouse show vein-clearing and downward cupping of the leaves about 5 days after inoculation. A mottle or mosaic (Fig. 1) develops after 15-20 days.- Gomphrena globosa. Chlorotic lesions in inoculated leaves within 4 days after
inoculation, soon becoming necrotic with red margins
(Fig. 2).
- Chenopodium amaranticolor. Chlorotic primary lesions within 7-10 days.
- Cassia occidentalis. Necrotic local lesions within 3-4 days.
- Chenopodium amaranticolor. Chlorotic primary lesions within 7-10 days.
Propagation species
- Papaya and snapdragon (Antirrhinum majus) are good hosts for maintenance of cultures and as sources of virus for purification.
Assay species
- Gomphrena globosa.
Strains
None distinguished.
Transmission by Vectors
No insect vector known. Conover (1964b) and Zettler, Edwardson & Purcifull (1968) tested several species of aphids.
Transmission through Seed
None reported.
Transmission by Dodder
No information.
Serology
The virus is strongly immunogenic. It reacts with antiserum to give flagellar precipitates in mixed liquids. Untreated sap from infected papaya or snapdragon plants gives positive results in agar-gel diffusion tests (de Bokx, 1965).
Relationships
Its physical and chemical properties are typical of viruses in the potato virus X group.
Stability in Sap
In papaya sap, the thermal inactivation point (10 min) is 73-76°C, the dilution end-point is about 10-4, and infectivity is retained after 6 months at room temperature (Conover, 1964b).
Purification
(Hiebert, unpublished). The virus is stable and is readily purified from systemically infected papaya leaves. Homogenize tissue (100 g) in a mixture of 100 ml buffer (0.02 M borate, pH 7.6, containing 0.5% sodium sulphite), 50 ml n-butanol and 50 ml chloroform. Centrifuge at low speed and collect the aqueous phase. Precipitate the virus by adding polyethylene glycol (‘Carbowax 6000’) to a final concentration of 0.5%. The virus is then subjected to 1 or 2 cycles of differential centrifugation. Yields are up to 3 mg virus per g of fresh tissue.
Papaya mosaic virus has also been purified from infected snapdragon (Antirrhinum majus) leaves using a similar procedure (Koenig et al., 1970).
Properties of Particles
Sedimentation coefficient (s20,w) at infinite dilution: 118.7 S (Hiebert, 1970).
Isoelectric point: c. pH 5.3.
Absorbance at 260 nm (1 mg/ml, 1 cm light path): 2.85 (Hiebert, 1970).
A260/A280: 1.40.
Electrophoretic mobility: -1 x 10-5 cm2 volt-1 sec-1 in 0.1 ionic strength sodium phosphate-buffered sodium chloride, pH 7.0, prepared according to Miller & Golder (1950).
Molecular weight (daltons): about 31.4 x 106, estimated from the RNA content and molecular weight.
Particle Structure
Particles are flexuous filaments (Fig. 3) about 530 nm long (de Bokx, 1965).
Particle Composition
RNA: M. Wt 2.2 x 106 (Koenig, 1971), single-stranded. Molar percentages of nucleotides: G20.7±0.2; A33.8±0.5; C23.4±0.4; U22.1±0.4. Phenol extracts yield one sedimenting species of RNA which is infectious and has a sedimentation coefficient of 31.8 S at infinite dilution in a solution containing 10-2 M Tris buffer (pH 7.4), 10-2 M KCl and 10-4 M MgCl2 (Hiebert, 1970). RNA is about 7% of the particle weight, estimated from the phosphorus content.
Protein: Electrophoretically homogeneous protein subunits were prepared by mild heating in Tris-borate buffer (pH 10) containing sodium dodecyl sulphate and 2-mercaptoethanol. Subunit M. Wt is 19.4±0.3 x 103 (Koenig et al., 1970).
Relations with Cells and Tissues
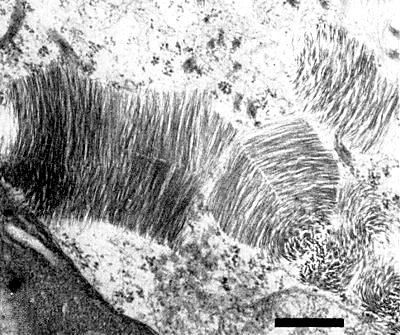
Cytoplasmic inclusions consisting of aggregated virus particles sectioned in various planes (Fig. 4) were observed in infected papaya leaf tissue (Zettler et al., 1968). These inclusions resemble those reported for clover yellow mosaic virus in pea (Purcifull, Edwardson & Christie, 1966).
Notes
Several serious mosaic diseases limit papaya production in various parts of the world, including India, Puerto Rico, South America, Hawaii and Florida. Among the mechanically transmissible viruses associated with these diseases, several differ from papaya mosaic virus in being aphid-borne and restricted in host range to papaya and cucurbits (Capoor & Varma, 1958; Conover, 1964a; Ishii & Holtzmann, 1963; Herold & Weibel, 1962). The best known of these viruses, papaya ringspot (Conover, 1964a), also differs from papaya mosaic virus because it has flexuous filamentous particles c. 780 nm long, is transmitted by aphids in the non-persistent manner, and induces cytoplasmic ‘pinwheel’ inclusions in host cells (Zettler et al., 1968).
Bunchy top disease of papaya is a serious problem in parts of the Caribbean area. A mycoplasma-like agent has been associated with this disease (Story & Halliwell, 1969).
Figures
References list for DPV: Papaya mosaic virus (56)
- Capoor & Varma, Indian J. agric. Sci. 28: 225, 1958.
- Conover, Phytopathology 52: 6, 1962.
- Conover, Proc. Fla St. hort. Soc. 77: 440, 1964a.
- Conover, Proc. Fla St. hort. Soc. 77: 444, 1964b.
- Cook & Zettler, Pl. Dis. Reptr 54: 893, 1970.
- de Bokx, Pl. Dis. Reptr 49: 742, 1965.
- Herold & Weibel, Virology 18: 302, 1962.
- Hiebert, Phytopathology 60: 1295, 1970.
- Ishii & Holtzmann, Pl. Dis. Reptr 47: 947, 1963.
- Koenig, J. gen. Virol. 10: 111, 1971.
- Koenig, Stegemann, Francksen & Paul, Biochim. biophys. Acta 207: 184, 1970.
- Miller & Golder, Archs Biochem. 29: 420, 1950.
- Purcifull, Edwardson & Christie, Virology 29: 276, 1966.
- Story & Halliwell, Phytopathology 59: 1336, 1969.
- Zettler, Edwardson & Purcifull, Phytopathology 58: 332, 1968.