Details of DPV and References
DPV NO: 79 October 1971
Family: Bromoviridae
Genus: Cucumovirus
Species: Tomato aspermy virus | Acronym: TAV
Tomato aspermy virus
M. Hollings Glasshouse Crops Research Institute, Littlehampton, Sussex, England
Olwen M. Stone Glasshouse Crops Research Institute, Littlehampton, Sussex, England
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by Blencowe & Caldwell (1949);
probably first isolated from chrysanthemum by
Ainsworth (1939).
Selected synonyms
- Chrysanthemum mosaic (Rev. appl. Mycol. 30: 231)
- Lycopersicon virus 7 (Rev. appl. Mycol. 29: 180)
- Chrysanthemum aspermy virus (Rev. appl. Mycol. 45: 2141)
- Cucumis virus 1 str. chr. (Rev. appl. Mycol. 32: 559)
- Lycopersicon virus 7 (Rev. appl. Mycol. 29: 180)
-
An RNA-containing virus with isometric particles c. 30 nm diameter. It has a wide host range, is readily transmitted by several species of aphid in the non-persistent manner, and by inoculation of sap. It is widespread and common in cultivated chrysanthemums but uncommon in tomatoes.
Main Diseases
Causes severe flower-break, dwarfing and distortion (Fig. 4) in cultivated chrysanthemums, but flower symptoms may not develop in the same year as infection. Most cultivars show no leaf symptoms and no loss of vegetative vigour (Brierley, 1955; Govier, 1957; Hollings, 1955; Noordam, 1952; Oertel, 1967). In tomato, causes severe leaf distortion and usually seedless fruits (Fig. 5). Causes mosaic in several dicotyledonous species and in Canna and Lilium.
Geographical Distribution
Prevalent in most countries where chrysanthemums are extensively grown, including W. Europe, Russia, USA, Canada, India, Japan, Australia and New Zealand.
Host Range and Symptomatology
The virus has a wide experimental host range, infecting over 100 species in 24 dicotyledonous and 3 monocotyledonous families, although at least 75 of these species are in the families Chenopodiaceae, Compositae and Solanaceae. The type strain, however, has a more restricted host range than do most isolates occurring in chrysanthemum. Systemic symptoms in most species are much more severe in winter than in summer.
-
Diagnostic species
- Nicotiana glutinosa.
Inoculated leaves may develop chlorotic or semi-necrotic spot or ring lesions, followed by systemic vein-clearing after 1-2 weeks. Severe light and dark green blister-mottle develops, with distortion and dwarfing (Fig. 1). In severe infections, tendril-like leaves are formed, and enations develop on the undersides of the leaves (Fig. 6). - N. tabacum and N. clevelandii develop severe systemic mottle, dwarfing and distortion;
although these species are more susceptible than N. glutinosa, the symptoms are less diagnostic.
- Cucumis sativus (cucumber). Most strains cause small local chlorotic spots in 3-8 days, in the cotyledons only; the true leaves are not infected, in contrast with cucumber mosaic virus. The type strain does not infect this species.
- Lycopersicon esculentum (tomato). Severe systemic leaf mottle, distortion and dwarfing, sometimes with proliferation of axillary shoots. Fruits are dwarfed, malformed and, with most strains of the virus, seedless (Fig. 5).
- Phaseolus vulgaris (French bean). In UK, minute pale necrotic local lesions develop during the winter months; not systemic. Not infected by type strain.
- Chenopodium amaranticolor and C. quinoa. Numerous chlorotic or necrotic local lesions (pin-point size in C. amaranticolor) develop in 3-7 days (Fig. 2, Fig. 3); not systemic.
- Vigna sinensis (cowpea). Local necrotic lesions develop in 2-4 days. Not infected by type strain.
- Cucumis sativus (cucumber). Most strains cause small local chlorotic spots in 3-8 days, in the cotyledons only; the true leaves are not infected, in contrast with cucumber mosaic virus. The type strain does not infect this species.
-
Propagation species
- Nicotiana glutinosa
is suitable for maintaining cultures but N. clevelandii is very much better as a source of virus for purification (Hollings, Stone & Brunt, 1968).Assay species
- Chenopodium amaranticolor,
C. quinoa and Vigna sinensis are useful local lesion hosts.
Strains
Many variants occur, differing considerably in host range, symptom severity, ease of transmission by aphids and stability in vitro.
Type strain (Blencowe & Caldwell, 1949). This has been in laboratory culture for over 20 years; it now has a very restricted host range and is more difficult to purify than typical chrysanthemum isolates (Hollings & Stone, 1969).
Most isolates from chrysanthemum cause seedless fruits in tomato, local lesions in cucumber cotyledons, and are serologically related to each other; however, some chrysanthemum isolates, serologically related to the type strain, did not induce seedless fruits in one cultivar of tomato (Lawson, 1967b).
A few isolates withstand heat treatment in vivo (see Notes).
Transmission by Vectors
Transmissible by 10 species of aphid (Kennedy, Day & Eastop, 1962), but differences between strains occur. Virus can be acquired in 15 sec and inoculated in less than 1 min. No latent period. Feeding vectors retain virus for 30 min, rarely 1 h (Brierley, Smith & Doolittle, 1955; Hollings, 1955). Some strains seem to have lost the ability to be transmitted by aphids.
Transmission through Seed
An isolate from Campanula rapunculoides was seed-borne in Stellaria media (Noordam et al., 1965). Not seed-borne in Nicotiana glutinosa, N. tabacum, Callistephus chinensis (Brierley et al., 1955), Chrysanthemum morifolium or Lycopersicon esculentum (Hollings, 1955).
Transmission by Dodder
Poorly transmitted by Cuscuta subinclusa and C. europea; not transmitted by three other Cuscuta spp. (Schmelzer, 1957).
Serology
The virus is a good immunogen. Antisera prepared by one intravenous and two intramuscular injections with Freund’s complete adjuvant, over a period of 3 weeks, had specific titres in precipitin tube tests up to 1/8000 (Hollings et al., 1968). Somatic (granular) precipitates are produced in precipitin tube and microprecipitin tests. Cucumber mosaic virus is reported to precipitate spontaneously with 0.85% NaCl (Francki et al., 1966); this does not occur with tomato aspermy virus. A single reaction line is produced in agar gel-diffusion tests (0.8% Ionagar No. 2 in 0.03 M phosphate buffer). In immunoelectrophoresis (0.03 M phosphate buffer, pH 7.6) the virus moves to the cathode. Serological tests have been used for detecting the virus in chrysanthemum. Gel-diffusion tests can be made with crude sap (Oertel, 1968) or with the sediment after low speed centrifugation of sap (Hakkaart, 1967). Microprecipitin tests can be made with clarified sap (Oertel, 1968).
Relationships
Even with narrow-spectrum antisera, most strains from chrysanthemum show strong cross-reactions; a few isolates react poorly or not at all with antisera to typical chrysanthemum strains; some chrysanthemum isolates are reportedly related to the type strain but not to other chrysanthemum isolates (Lawson, 1967a). Broad-spectrum antisera extend the range of cross-reactions (Hollings et al., 1968). Much, however, depends on the method of antiserum production; low-titred antisera have sometimes been used.
Reports differ on the relationship between tomato aspermy and cucumber mosaic viruses; relationships have been found with some isolates of tomato aspermy virus (Govier, 1957; Roland, 1959; Lawson, 1967a) but not with others (Grogan, Uyemoto & Kimble, 1963; Oertel, 1969; Tochihara, 1970). Broad-spectrum antisera to tomato aspermy virus and to some cucumber mosaic virus strains react with the heterologous viruses in conditions when narrow-spectrum antisera do not (Hollings et al., 1968).
Similar variations occur in plant protection tests; full, partial or no protection occurs depending on the strains of tomato aspermy virus and cucumber mosaic virus used, the order of inoculation, the test plant, and the season of the year (Brierley et al., 1955; Hollings, 1955; Govier, 1957; Noordam, 1952; Graham, 1957; Holmes, 1956; Inouye, Asatani & Mitsuhata, 1968; Noordam et al., 1965). Strains of cucumber mosaic virus and tomato aspermy virus that are serologically related do not necessarily show cross-protection in plants, nor has a serological relationship been shown between all strains that cross-protect in plants.
Stability in Sap
Minor differences have been reported for different strains or isolates. Infectivity in tobacco sap is lost after 10 min at 50, 55 or 60°C, depending on virus strain and initial concentration. Dilution end-point in tobacco sap is about 10-4 to 10-5; in Nicotiana clevelandii sap 10-5 to 10-6; in chrysanthemum sap 10-2 to 10-3. Longevity in vitro is 2-6 days at 20°C, or 9-12 months at -5°C. Infectivity survives at least 3 years in shredded leaves at -5°C and at least 11 years when lyophilized and stored under high vacuum at 18°C (Hollings & Stone, 1970).
Purification
Most strains are very much more readily purified than cucumber mosaic virus strains. Several methods are satisfactory.
1. Modification of Steere’s method (Marani, 1969). Harvest tobacco plants 6 weeks after inoculation, and store 15 days at -20°C. Mince leaves and express the juice through cloth. Add two volumes of a 1:1 mixture of n-butanol and chloroform slowly to the juice at 4°C and stir vigorously for 15 min. Centrifuge the emulsion 30 min at c. 1000 g. Store the aqueous (top) layer 12-16 hr at 22°C, then centrifuge 30 min at 8000 rev/min (c. 10,000 g) and separate the virus from the supernatant fluid by several cycles of differential centrifugation. Resuspend the final pellets in distilled water and centrifuge briefly to remove insoluble material.
2. Grogan et al. (1963), modified by Lawson (1967a). Harvest Samsun tobacco plants, grown in shade, 10-14 days after inoculation, and grind in 1.5 vol of 0.3 M phosphate buffer (pH 7.6) containing 0.01 M sodium diethyl dithiocarbamate (DIECA) and 0.005 M L-cysteine. Store the extract overnight at c. 4°C, then add 6 N HCl dropwise to bring the pH to 4.6 and stir the mixture for 30 min. Centrifuge at low speed and retain the supernatant fluid. Concentrate the virus by one cycle of differential centrifugation, and resuspend the final pellets in 0.1 M phosphate buffer, followed by brief centrifugation to remove insoluble material.
3. Hollings et al. (1968).
Harvest Nicotiana clevelandii plants 10-14 days after
inoculation and homogenize each 100 g leaf material at laboratory temperature with 200 ml 0.05 M
phosphate buffer containing 0.1% thioglycollate; express the juice. Add n-butanol to 8.5%
(v/v) of the final volume and stand the mixture overnight at c. 4°C. Separate the virus
by one or more cycles of differential centrifugation, resuspend the final pellets in 0.02 vol 0.03
M phosphate buffer, and after 2-3 hr remove insoluble material by brief centrifugation. Yields may
be up to 25 mg virus per 100 g leaf tissue extracted.
Further purification can be obtained by density gradient centrifugation followed by dialysis of the virus from the light-scattering zone against 200 vol 0.01 M phosphate buffer containing 0.01 M NaCl. The yield of virus depends greatly on the species of host plant, the time after inoculation and on leaving the butanol-treated homogenate for 12-24 hr at 4°C to free the virus from plant material.
Properties of Particles
Sedimentation coefficient (s20, w) at infinite dilution: 98-100 S.
A260/A280: 1.73-1.77 (R. Stace-Smith, unpublished).
Particle Structure
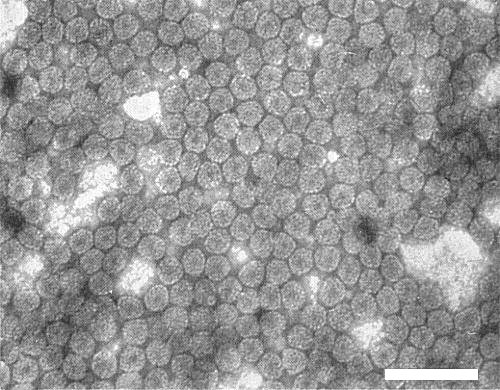
Particles are isometric, 25-30 nm diameter (Fig. 7) and are more stable in phosphotungstate than those of cucumber mosaic virus (Hollings et al., 1968; Tochihara, 1970). In thin sections, particles measured 23-25 nm (Lawson & Hearon, 1970).
Particle Composition
RNA: Probably single-stranded. Molar percentages of nucleotides: G23.7; A26.4; C21.2; U28.7 (R. Stace-Smith. unpublished).
Protein: No information.
Other components: None reported.
Relations with Cells and Tissues
All tissues can be infected. No inclusion bodies are reported, although aggregates of virus particles have been seen in ultrathin sections of leaf parenchyma. Particles are present in plasmodesmata, and between the cisternae of dictyosomes (Lawson & Hearon, 1970). The virus interferes with normal meiotic division in Nicotiana glutinosa and some other members of the Solanaceae (Wilkinson, 1953). The histology of enations has been studied (Praceus, 1958).
Notes
Tomato aspermy and cucumber mosaic viruses have many similarities, and some strains from each group are serologically related and cross-protect in plants. The distinguishing features of the two viruses tend to overlap but most strains of tomato aspermy virus differ from most strains of cucumber mosaic virus in that they (i) infect only the cotyledons of cucumber; (ii) induce seedless (or nearly seedless) fruits in tomato; (iii) cause enations on the undersides of leaves of several Nicotiana species; and (iv) infect Chrysanthemum morifolium.
Indexing chrysanthemums is best done by inoculating N. glutinosa plants with sap from young shoots or flowers; serological testing has been developed by Hakkaart (1967) and Oertel (1968).
Most strains of the virus are eliminated from chrysanthemums after c. 4 weeks at 37°C (Hollings & Kassanis, 1957; Brierley & Lorentz, 1960) or by meristem-tip culture (Dunez & Monsion, 1968). A few heat-tolerant isolates have been found in Britain, able to persist at 37°C, but their other properties were similar to those of typical strains.
Figures

Systemic symptoms in Nicotiana glutinosa, 3 weeks after infection, showing chlorotic mottle and beginning of leaf distortion.

Flowers of chrysanthemum cv. Cherry Favourite: (left) virus-free and (right) infected, showing dwarfing, colour break and distortion.

Transverse section of tomato fruits: (below) healthy; and (above) completely seedless fruit from infected plant.
References list for DPV: Tomato aspermy virus (79)
- Ainsworth, Rep. exp. Res. Stn Cheshunt, 1938: 60, 1939.
- Blencowe & Caldwell, Ann. appl. Biol. 36: 320, 1949.
- Brierley, Phytopathology 45: 2, 1955.
- Brierley & Lorentz, Phytopathology 50: 404, 1960.
- Brierley, Smith & Doolittle, Pl. Dis. Reptr 39: 152, 1955.
- Dunez & Monsion, Annls Épiphyt. 19: 165, 1968.
- Francki, Randles, Chambers & Wilson, Virology 28: 729, 1966.
- Govier, Ann. appl. Biol. 45: 62, 1957.
- Graham, Virology 3: 427, 1957.
- Grogan, Uyemoto & Kimble, Virology 21: 36, 1963.
- Hakkaart, Neth. J. Pl. Path. 73: 181, 1967.
- Hollings, Ann. appl. Biol. 43: 86, 1955.
- Hollings & Kassanis, Jl. R. hort. Soc. 82: 339, 1957.
- Hollings & Stone, Rep. Glasshouse Crops Res. Inst. 1968: 103, 1969.
- Hollings & Stone, Ann. appl. Biol. 65: 411, 1970.
- Hollings, Stone & Brunt, Rep. Glasshouse Crops Res. Inst. 1967: 95, 1968.
- Holmes, Virology 2: 611, 1956.
- Inouye, Asatani & Mitsuhata, Nogaka Kenkyu 52: 55, 1968.
- Kennedy, Day & Eastop, A conspectus of aphids as vectors of plant viruses, London, Commonwealth Institute of Entomology, 1962.
- Lawson, Virology 32: 357, 1967a.
- Lawson, Pl. Dis. Reptr 51: 723, 1967b.
- Lawson & Hearon, Virology 41: 30, 1970.
- Marani, Phytopath. Mediterranea 8: 142, 1969.
- Noordam, Tijdschr. PlZiekt. 58: 121, 1952.
- Noordam, Bijl, Overbeek & Quiniones, Neth. J. Pl. Path. 71: 57, 1965.
- Oertel, Zentbl. Bakt. ParasitKde Abt. II 121: 276, 1967.
- Oertel, Z. PflKrankh. PflPath. PflSchutz 75: 605, 1968.
- Oertel, Nova Acta Leopoldina 189: 1, 1969.
- Praceus, Phytopath. Z. 33: 248, 1958.
- Roland, Parasitica 15: 43, 1959.
- Schmelzer, Phytopath. Z. 30: 449, 1957.
- Tochihara, Ann. phytopath. Soc. Japan 36: 1, 1970.
- Wilkinson, Ann. Bot. N.S. 17: 219, 1953.