Details of DPV and References
DPV NO: 81 June 1972
Family: Secoviridae
Genus: Fabavirus
Species: Broad bean wilt virus 1 | Acronym: BBWV-1
Broad bean wilt virus
R. H. Taylor Victorian Plant Research Institute, Burnley, Victoria, Australia
L. L. Stubbs University of Melbourne, Parkville, Victoria, Australia
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by Stubbs (1947,
1960).
Synonyms
- Nasturtium ringspot virus (Rev. appl. Mycol. 30: 40)
- Petunia ringspot virus (Rev. appl. Mycol. 39: 713)
- P.O. pea streak virus (Rev. appl. Mycol. 39: 362)
- Ringmosaik-Virus der Kapuzinerkresse (Rev. appl. Mycol. 40: 51)
- Parsley virus 3 (Rev. Pl. Path. 49: 35551)
- Petunia ringspot virus (Rev. appl. Mycol. 39: 713)
-
An RNA-containing virus with isometric particles about 25 nm in diameter. It has a wide host range, is transmitted by at least three species of aphid in the non-persistent manner and is readily transmissible by inoculation of sap. Widely distributed throughout the world but of minor economic importance.
Main Diseases
Wilt of broad beans (Stubbs, 1947; 1960), streak of peas (Kim & Hagedorn, 1959), blight of spinach (Schroeder & Provvidenti, 1970), ringspot or ring- mosaic of nasturtium (Smith, 1950; Schmelzer, 1960) and ringspot of petunia (Rubio, 1959). Also found naturally in Digitalis lanata (Schumann, 1963), Catalpa bignonioides and carrot (Schmelzer & Wolf, 1969), parsley (Wolf, 1970; Frowd & Tomlinson, 1970) and Capsicum annuum (M. Conti & G. Boccardo, pers. comm.).
Geographical Distribution
Southeastern Australia, New York State (USA), Japan (Y. Komuro, pers. comm.), Europe.
Host Range and Symptomatology
Host range is wide. The broad bean wilt isolate infected 43 species in 14 dicotyledonous families by inoculation of sap (Stubbs, 1947; R. H. Taylor & P. R. Smith, unpublished); an isolate from nasturtium infected 63 out of 125 species in 19 dicotyledonous families (Schmelzer, 1960). The only monocotyledonous host reported is Narcissus (Y. Komuro, pers. comm.). Ringspots are characteristic symptoms of infection, especially in solanaceous species.
-
Diagnostic species
- Vicia faba
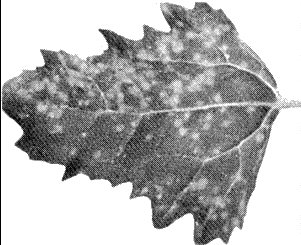
(broad bean). Systemic vein-clearing, rapidly followed by necrosis of terminal leaves (Fig. 2), general wilting and often death. May partially recover; fully turgid ‘recovered’ plants have cupped distorted leaves with dark green mosaic mottle. The P.O. strain induces local lesions in 4-5 days (Fig. 3) followed by systemic mottle and necrosis. The parsley and nasturtium strains induce only mild symptoms in broad bean (J. A. Frowd, pers. comm.). The petunia ringspot strain infects this species less readily and gives less prominent symptoms (T. Doel, pers. comm.). - Datura stramonium
(Fig. 4).
Concentric necrotic ring-shaped local
lesions which coalesce; inoculated leaves may absciss. Chlorotic or necrotic
ringspots and line or oak-leaf patterns on new growth. The petunia ringspot
strain infects this species less readily and gives less prominent symptoms (T.
Doel, pers. comm.).
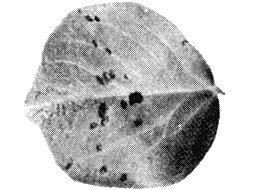
- Chenopodium quinoa. Chlorotic local lesions in inoculated leaves (4-5 mm diameter) after 4-6 days (Fig. 1), followed by systemic chlorosis of the whole plant or of the apical leaves with epinasty and necrotic streaks. Plants die as a result of infection by the nasturtium isolate.
- Nicotiana tabacum cv. White Burley. Chlorotic ringspots in inoculated leaves, extending up to 8 mm diameter and becoming necrotic. Inconspicuous systemic reaction.
- Chenopodium quinoa. Chlorotic local lesions in inoculated leaves (4-5 mm diameter) after 4-6 days (Fig. 1), followed by systemic chlorosis of the whole plant or of the apical leaves with epinasty and necrotic streaks. Plants die as a result of infection by the nasturtium isolate.
-
Propagation species
- Broad bean cv. Cole’s Prolific is suitable for maintaining cultures of the broad bean strain and as a source of virus for purification. After the initial necrotic reaction the concentration of virus falls, but some virus may be recovered throughout the life of the plant. With nasturtium, parsley and petunia ringspot strains, Nicotiana clevelandii is a suitable host for maintaining cultures and Chenopodium quinoa is a good source of virus for purification.
-
Assay species
- Vigna sinensis
(cowpea) is a good local lesion host for the broad bean wilt strain; reddish-brown local lesions develop 3-4 days after inoculation. Chenopodium amaranticolor and C. quinoa are much more sensitive local lesion hosts for the nasturtium, parsley and petunia ringspot strains.
Strains
The most important strains are:
The type strain of Stubbs (1947). Source Australia. After numerous transfers in broad bean this strain lost ability to cause much necrosis, indicating that strain selection had occurred. All Australian isolates produce identical symptoms in broad bean.
The P.O. pea streak virus of Kim & Hagedorn (1959). Source USA. Differs from the type strain in producing local lesions followed by systemic mosaic in broad bean; also differs in other host reactions (W. T. Schroeder, pers. comm.).
The nasturtium ringspot strain of Smith (1949a, 1949b, 1950). Source UK. The parsley isolate of Frowd & Tomlinson (1970) may belong to this strain. Less virulent than the type strain in broad bean.
The Petunia ringspot strain of Rubio (1959). Source Spain. Less virulent than the type strain in broad bean and Datura stramonium.
Transmission by Vectors
Transmissible by the aphids Myzus persicae, Aphis craccivora and Macrosiphum euphorbiae. M. persicae is the most efficient vector (Stubbs, 1960). Nasturtium isolates were also transmitted by Aphis fabae (Smith, 1950) and Acyrthosiphon onobrychis (Schmelzer, 1960).
Transmission through Seed
Seed from infected broad bean plants produces healthy progeny. No seed transmission in nasturtium (Schmelzer, 1960).
Transmission by Dodder
Not transmitted by Cuscuta californica, C. campestris or C. subinclusa (Schmelzer, 1960).
Serology
The virus is strongly immunogenic. Antisera with titres of 1/1024-l/2048 were produced in rabbits receiving one intramuscular injection with adjuvant, followed by two intravenous injections (Taylor et al., 1968). In gel- diffusion tests using purified preparations one sharp curved band of precipitate forms.
Poorly developed bands of precipitate are obtained with sap from infected broad bean plants unless leaves are ground in buffer containing a reducing agent (for each 1 g of tissue use 1 ml 0.05 M phosphate buffer containing 0.1% thioglycollate + 0.1 M diethyldithiocarbamate at pH 7.6).
Relationships
The type and P.O. strains are serologically indistinguishable in gel-diffusion tests (Taylor et al., 1968). Recent work (S. M. Cook & A. J. Gibbs, pers. comm.; Frowd & Tomlinson, 1970; Murant & Goold, 1972; T. Doel, pers. comm.) indicates that nasturtium ringspot virus, petunia ringspot virus and an isolate from parsley are also serologically closely related to, or indistinguishable from, broad bean wilt virus. Nasturtium mosaic virus (Jensen, 1950) may also be related. Broad bean wilt virus has some similarities to viruses in the cowpea mosaic virus group (Gibbs et al., 1966, 1968). However, in gel-diffusion tests it did not react with antisera prepared against the following viruses of this group: broad bean stain, red clover mottle, squash mosaic or Echtes Ackerbohnemosaik (Taylor et al., 1968).
Stability in Sap
In broad bean sap, the thermal inactivation point (10 min) is c. 58°C, dilution end-point 10-4-10-5 and infectivity is retained at 21°C for 2-3 days (Stubbs, 1947; Schmelzer, 1960).
Purification
The virus is readily purified; broad beans infected with the broad bean wilt strain, harvested just before the onset of necrosis, usually yield 1 mg virus per 100 g tissue. Chenopodium quinoa may yield up to 10 mg virus per 100 g tissue (T. Doel, pers. comm.).
To purify the broad bean wilt strain, inoculate broad bean seedlings 2-3 days after emergence. Harvest 10-14 days later; immediately cool and homogenize in phosphate buffer (0.1 M, pH 7.6) containing diethyl-dithiocarbamate (0.1 M) and thioglycollate (0.1%). Keep homogenate overnight at 4°C, filter through muslin, emulsify with chloroform (quarter volume) and centrifuge (10,400 g for 20 min). Collect aqueous phase and purify virus by three cycles of differential centrifugation. Resuspend the pellets in phosphate buffer (0.05 M, pH 7.6) with or without 0.01 M EDTA. The virus can be further purified and fractionated in sucrose density gradients (Taylor et al., 1968).
The following procedure was useful with the parsley and nasturtium isolates (Frowd, 1971): Harvest systemically infected C. quinoa plants 12-14 days after inoculation, homogenize in 0.05 M phosphate buffer (pH 7.5) containing 1 mM ethylenediamine-tetraacetic acid (sodium salt) and 0.15% 2-mercaptoethanol. Filter through muslin, stir 45 min with 8.5%, (v/v) n-butanol. Centrifuge at 2200 g for 15 min and collect supernatant fluid. Centrifuge at 66,000 g for 90 min. To minimize virus aggregation, resuspend in distilled water (Frowd, 1971).
Properties of Particles
Purified preparations contain three sorts of particles: empty protein shells without RNA (T), and two sorts of nucleoprotein particles containing different amounts of RNA (M and B).
Sedimentation coefficients (s20,w) at infinite dilution (svedbergs): 63 (T), 100 (M) and 126 (B).
A260/A280: 1.32 (T), 1.64 (M), 1.75 (B).
Particle Structure
Particles are isometric, about 25 nm in diameter (Fig. 5); they are stable in phosphotungstate and many are obviously hexagonal in shape, but subunits are not obvious. Some of the M and B particles, and all of the T particles are penetrated by phosphotungstate. The particles are stable in uranyl acetate, pH 4.7.
Particle Composition
RNA: probably single-stranded. The T, M and B particles contain 0, 22 and 33% RNA respectively. Infectivity seems to be almost entirely associated with the B particles. Molar percentages of nucleotides for the broad bean wilt isolate are G25; A30; C18; U27; similar results were obtained for nasturtium ringspot and petunia ringspot isolates (T. Doel, pers. comm.).
Protein: particles contain a polypeptide of M. Wt c. 40,000 (Carpenter, Cook & Gibbs, 1971). T. Doel (pers. comm.) found, for the broad bean wilt isolate, two polypeptides of about 42,000 and 26,000 M. Wt; similar results were obtained for nasturtium ringspot and petunia ringspot isolates.
Relations with Cells and Tissues
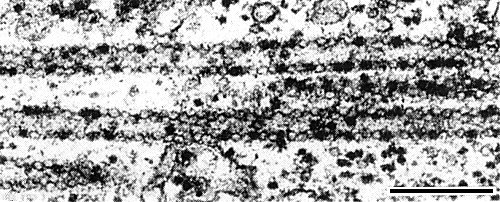
In epidermal cells of broad bean, the petunia ringspot strain induces amorphous and crystalline inclusions visible by light microscopy (Rubio-Huertos, 1962). In cells of Vicia faba infected with petunia ringspot virus, and of Chenopodium amaranticolor and Nicotiana clevelandii infected with an isolate from nasturtium, virus particles occur in tubular arrays (Fig. 6, Fig. 7); in cross sections of the tubes nine particles can usually be seen (Rubio-Huertos, 1968; Sahambi, Milne & Phillips, 1970, and unpublished). In cells of Chenopodium quinoa infected with an isolate from parsley, virus particles were packed into crystalline arrays, scrolls and simple tubes (Frowd, 1971; Hull & Plaskitt, 1971). These structures were not found in cells infected with broad bean wilt virus (R. G. Milne, pers. comm.) or with the type culture of nasturtium ringspot virus (Frowd, 1971; Hull & Plaskitt, 1971). With the petunia ringspot strain, particles also formed hexagonally packed sheets, often lying between adjacent mitochondria, and loose networks of interlocking rings; virus-like particles were also found in tubules passing through cell walls (Hull & Plaskitt, 1971).
Notes
In New South Wales the virus is present in weed hosts but does not appear to cause any diseases of economic plants. In Victoria, the disease appears to be restricted to broad beans in urban localities and may be less prevalent now than in the past. In USA (Schroeder & Provvidenti, 1970) and Japan (Y. Komuro, pers. comm.) it causes a severe disease of spinach which may be confused with that caused by cucumber mosaic virus. The particles of broad bean wilt virus closely resemble those of viruses of the cowpea mosaic virus group in size, appearance, sedimentation behaviour, nucleic acid content and composition; however, broad bean wilt virus is transmissible by aphids, whereas viruses of the cowpea mosaic virus group are transmissible by beetles or have no known vectors.
Figures

Local and systemic symptoms in Datura stramonium. Note that local lesions may develop into concentric necrotic rings.
References list for DPV: Broad bean wilt virus (81)
- Carpenter, Cook & Gibbs, Rep. Rothamsted exp. Stn 1970: 122, 1971.
- Frowd, Ph.D. Thesis, University of Birmingham, 1971.
- Frowd & Tomlinson, Pl. Dis. Reptr 54: 734, 1970.
- Gibbs, Hecht-Poinar, Woods & McKee, J. gen. Microbiol. 44: 177, 1966.
- Gibbs, Giussani-Belli & Smith, Ann. appl. Biol. 61: 99, 1968.
- Hull & Plaskitt, Rep. John Innes Inst. 1970: 69, 1971.
- Jensen, Phytopathology 40: 967, 1950.
- Kim & Hagedorn, Phytopathology 49: 656, 1959.
- Murant & Goold, Rep. Scott. hort. Res. Inst. 1971: 64, 1972.
- Rubio, Microbiol. esp. 12: 325, 1959.
- Rubio-Huertos, Microbiol. esp. 15: 1, 1962.
- Rubio-Huertos, Protoplasma 65: 465, 1968.
- Sahambi, Milne & Phillips, Rep. Rothamsted exp. Stn 1969: 143, 1970.
- Schmelzer, Z. PflKrankh. PflPath. PflSchutz 67: 193, 1960.
- Schmelzer & Wolf, Zentbl. Bakt. ParasitKde Abt. 2 123: 577, 1969.
- Schroeder & Provvidenti, Phytopathology 60: 1405, 1970.
- Schumann, Phytopath. Z. 48: 135, 1963.
- Smith, Jl R. hort. Soc. 74: 482, 1949a.
- Smith, Jl R. hort. Soc. 74: 521, 1949b.
- Smith, Jl R. hort. Soc. 75: 350, 1950.
- Stubbs, J. Dep. Agric. Vict. 46: 323, 1947.
- Stubbs, Aust. J. agric. Res. 11: 734, 1960.
- Taylor, Smith, Reinganum & Gibbs, Aust. J. biol. Sci. 21: 929, 1968.
- Wolf, Acta phytopath. Acad. Sci. hung. 5: 95, 1970.