Details of DPV and References
DPV NO: 103 October 1972
Family: Secoviridae
Genus: Nepovirus
Species: Grapevine chrome mosaic virus | Acronym: GCMV
Grapevine chrome mosaic virus
G. P. Martelli Istituto di Patologia Vegetale, Università di Bari, 70126 Bari, Italy
A. Quacquarelli Istituto di Patologia Vegetale, Università di Bari, 70126 Bari, Italy
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by Martelli, Lehoczky & Quacquarelli (1965) and Martelli &
Quacquarelli (1972).
- Synonym
- Hungarian grapevine chrome mosaic virus (Rev. appl. Mycol. 48,
3084).
- A virus with isometric particles c. 30 nm in diameter, which sediments as two components containing 40 or 31% single-stranded RNA. Probably soil-borne but vector unknown. Readily transmitted by inoculation of sap to a moderate range of hosts. It occurs in Europe.
Main Diseases
Causes chrome-yellow mosaic (Fig. 1), stunting and decline of grapevine; it may also induce fanleaf symptoms. An isolate of the virus is associated with celery yellow-vein disease (Hollings, Stone & Martelli, 1969).
Geographical Distribution
Reported from Hungary and England.
Host Range and Symptomatology
Transmitted experimentally to 14 species in 5 dicotyledonous families (Martelli et al., 1965; Martelli & Quacquarelli, 1972; Hollings & Stone, 1970). Susceptible solanaceous plants usually remain symptomless.
- Diagnostic species
- Chenopodium quinoa. Small chlorotic local lesions, sometimes with
necrotic centres (Fig. 2) followed by conspicuous systemic chlorosis, necrotic
speckling and apical necrosis.
- Gomphrena globosa. Local lesions at first chlorotic, then reddish; transient systemic mosaic and vein-clearing.
- Datura stramonium. Inoculated leaves symptomless. Transient yellow zonate spots appear in systemically infected leaves.
- Phaseolus vulgaris (French bean). No symptoms in inoculated leaves. Systemic mosaic, yellow-green rings and chlorotic specks (Fig. 4) and, rarely, necrotic blotches.
- Gomphrena globosa. Local lesions at first chlorotic, then reddish; transient systemic mosaic and vein-clearing.
- Propagation species
- Phaseolus vulgaris is a good source of virus for purification.
- Assay species
- Chenopodium quinoa, although not very sensitive, can be used for local lesion assays.
Strains
Only minor variants differing in virulence towards herbaceous hosts have been distinguished.
Transmission by Vectors
The vector is not known but the virus is probably soil-borne; the nematode Xiphinema vuittenezi has been found associated with spread of the disease in Hungary (Martelli & Sàrospataki, 1969).
Transmission through Seed
Not tested.
Transmission by Dodder
Not tested.
Serology
The virus is a moderately good immunogen; antisera with titres up to 1/2048 have been obtained (Hollings et al., 1969). Precipitates in tube tests are granular. A single distinct precipitin band forms in gel-diffusion tests.
Relationships
Distantly related serologically to cocoa necrosis virus (Kenten, 1972). In biological, morphological, and physico-chemical properties it resembles members of the nepovirus group but no serological relationship to any member of the group has been detected (Hollings et al., 1969; Martelli & Quacquarelli, 1972).
Stability in Sap
In bean sap infectivity is lost after dilution to 10-3-10-4, heating for 10 min at 60-62°C, or storing for 1 week at 22°C (Martelli & Quacquarelli, 1972).
Purification
A modification of Steere’s chloroform-butanol method, followed by density gradient centrifugation, is useful (Martelli & Quacquarelli, 1972).
Properties of Particles
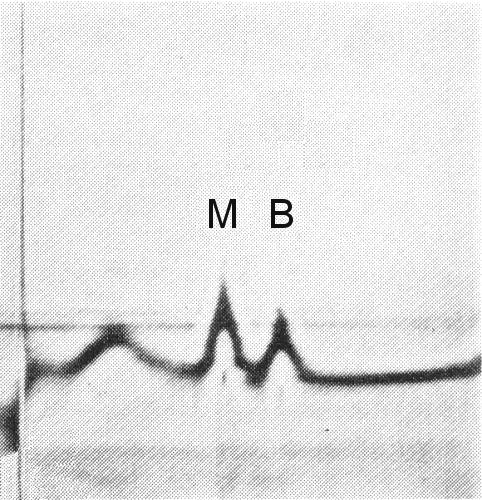
The particles are all the same size but sediment as two components (M and B) (Fig. 3), both containing RNA (Martelli, 1965; Martelli & Quacquarelli, 1972). No RNA-free (top) component has been observed.
Sedimentation coefficients (s20,w) (svedbergs): 92 (M)
and 117 (B).
Buoyant density in CsCl at 25°C: 1.416 (M) and 1.486 (B).
Particle Structure
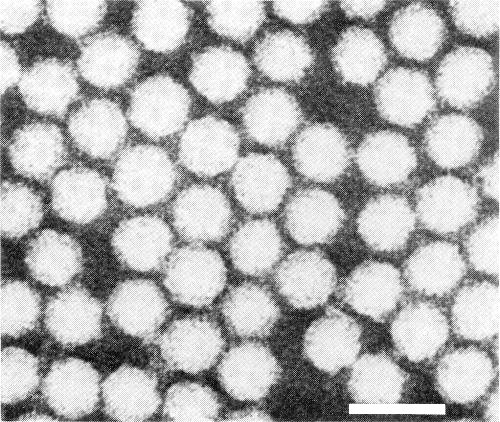
Particles are isometric, c. 30 nm in diameter, with angular outlines (Fig. 5). Some particles are penetrated by neutral sodium phosphotungstate.
Particle Composition
RNA: Single-stranded; c. 31% (M) and 40% (B) of the particle weight (estimated from buoyant density).
Protein. c. 69% (M) and 60% (B) of the particle weight. Molecular weight of particle polypeptides and amino acid composition unknown.
Relations with Cells and Tissues
The nitrogen metabolism of leaves of infected grapevines is disturbed (Jàkò, Lehoczky & Sàrospataki, 1966), the pigment and sugar contents affected (Jàkò et al., 1968) and photosynthetic CO2 fixation greatly diminished (Pozsàr et al., 1969).
Notes
In the field, grapevines affected by the chrome mosaic disease are difficult to distinguish from those with yellow mosaic. However, under shady glasshouse conditions, they continue to develop symptoms on the newly formed leaves, whereas plants with yellow mosaic do not. Grapevine chrome mosaic virus is difficult to distinguish from tomato black ring virus and other nepoviruses by symptoms in herbaceous hosts. Serological tests provide the only reliable means of identification.
Figures
References list for DPV: Grapevine chrome mosaic virus (103)
- Hollings & Stone, Rep. Glasshouse Crops Res. Inst. 1969: 129, 1970.
- Hollings, Stone & Martelli, Rep. Glasshouse Crops Res. Inst. 1968: 102, 1969.
- Jàkò, Lehoczky & Sàrospataki, Acta phytopath. Acad. Sci. Hung. 1: 185, 1966.
- Jàkò, Muranyi, Sàrospataki & Lehoczky, Acta phytopath. Acad. Sci. Hung. 3: 165, 1968.
- Kenten, Ann. appl. Biol. 71: 119, 1972.
- Martelli, Proc. int. Conf. Virus Vector Peren. Hosts, Univ. Cal. Agr. Sci., Davis: 402, 1965.
- Martelli & Quacquarelli, Annls Phytopath. numéro hors série: 13, 1972.
- Martelli & Sàrospataki, Phytopath. Mediterranea 8: 1, 1969.
- Martelli, Lehoczky & Quacquarelli, Proc. int. Conf. Virus Vector Peren. Hosts, Univ. Cal. Agr. Sci., Davis: 389, 1965.
- Pozsàr, Horvàth, Lehoczky & Sàrospataki, Vitis 8: 206, 1969.