Details of DPV and References
DPV NO: 104 October 1972
Family: Potyviridae
Genus: Potyvirus
Species: Pepper veinal mottle virus | Acronym: PVMV
Pepper veinal mottle virus
A. A. Brunt Glasshouse Crops Research Institute, Littlehampton, Sussex, England
R. H. Kenten Rothamsted Experimental Station, Harpenden, Hertfordshire, England
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by Brunt & Kenten (1971).
- An RNA-containing virus with filamentous particles usually flexuous and c. 770 x 12 nm, but under some conditions straight and c. 850 x 12 nm. It is readily sap-transmissible to a narrow range of hosts, and is transmitted by aphids in the non-persistent manner. A member of the potato virus Y group of viruses. Prevalent in Capsicum annuum and C. frutescens in Ghana.
Main Diseases
Causes severe leaf chlorosis in Petunia hybrida, and leaf mottling, severe leaf distortion and considerable loss of yield in naturally infected Capsicum annuum and C. frutescens (Fig. 1).
Geographical Distribution
Reported only from Ghana.
Host Range and Symptomatology
Of 16 host species, 11 are members of the Solanaceae; 5 of 46 species tested, from 3 of 17 other families, are also susceptible.
- Diagnostic species
- Capsicum annuum
(pepper) cv. Long Red. Conspicuous systemic vein chlorosis within 14-21 days, soon followed by interveinal chlorosis and general mottling (Fig. 2). - Chenopodium amaranticolor. Local chlorotic and semi-necrotic lesions
c. 1-2 mm in diameter after 10-14 days (Fig. 4); no systemic infection.
- Petunia hybrida cv. Rosy Morn. Faintly chlorotic local lesions, followed by conspicuous systemic leaf mottling.
- Nicotiana clevelandii. Inoculated leaves usually symptomless; systemically infected leaves become severely chlorotic within 21 days.
- Nicotiana megalosiphon. Severe systemic leaf chlorosis (Fig. 3).
- Nicotiana tabacum cvs. Dutch A and White Burley. Circular chlorotic lesions in inoculated leaves; no systemic infection.
- Petunia hybrida cv. Rosy Morn. Faintly chlorotic local lesions, followed by conspicuous systemic leaf mottling.
- Propagation species
- Cultures may be maintained in Nicotiana clevelandii, N. megalosiphon
or Petunia hybrida, and all three species are good sources of inoculum
and of virus for purification.
- Assay species
- Chenopodium amaranticolor
and C. quinoa are sensitive local lesion assay hosts.
Strains
None described.
Transmission by Vectors
Transmitted efficiently in the non-persistent manner by Myzus persicae and Aphis gossypii. Virus can be acquired and inoculated in 2 min feeding periods. Pepper veinal mottle virus resembles potato virus A and potato virus Y (Kassanis & Govier, 1971) in aiding the aphid transmission of potato aucuba mosaic virus (Brunt & Kenten, 1971).
Transmission through Seed
Not seed-borne in Capsicum annuum, Nicotiana megalosiphon or N. clevelandii (Brunt & Kenten, 1971).
Transmission by Dodder
Not attempted.
Serology
The virus is a good antigen; antisera react in tube precipitin tests to produce flagellar precipitates at dilutions up to 1/8000 (Brunt & Kenten, 1971). The antiserum fails to react with intact virus in gel-diffusion tests, but produces a single line of precipitate with disrupted virus.
Relationships
The virus has many properties in common with potato Y and allied viruses. However, it is serologically unrelated to tobacco etch, potato Y, turnip mosaic and 11 other morphologically similar viruses (Brunt & Kenten, 1971), and thus seems to be a distinct member of the potyvirus group.
Stability in Sap
In Capsicum annuum sap, the thermal inactivation point (10 min) is 55-60°C, dilution end-point 10-3-10-4, and infectivity is retained at 25°C for c. 7-8 days. Sap lyophilized with 7% peptone and 7% dextrose is still infective after 7 years in vacuo.
Purification
High molarity buffers prevent adsorption of virus to normal plant constituents. Chloroform, ether or carbon tetrachloride clarify sap without deleterious effects, but the virus particles are fragmented by n-butanol. Yields of 12-15 mg virus can be consistently obtained from 1 kg leaf tissue of Nicotiana clevelandii, N. megalosiphon or Petunia hybrida by the following procedure (Brunt & Kenten, 1971). Homogenize systemically infected leaves (1 g: 1 ml: 2 ml) in chloroform and 0.5 M borate at pH 7.8 containing 0.2% 2-mercaptoethanol; centrifuge at 10,000 g for 10 min, and precipitate virus from the aqueous phase by adding 50 g polyethylene glycol (M. Wt 6000) to each litre of the extract and stirring for 1-2 hr at 2°C. Resuspend virus in 0.5 M borate at pH 7.8 for 2 hr, then clarify and concentrate by differential centrifugation. The virus is further purified by reprecipitation with polyethylene glycol followed by several cycles of differential centrifugation, or by extracting and concentrating zones formed during centrifugation in 10-40% sucrose density gradients. Purified virus preparations in 0.05 M borate at pH 7.8 are mainly monodispersed with few impurities, and remain infective and unaggregated for at least 1 month at 2°C.
Properties of Particles
Sedimentation coefficient (s°20,w): 155 S. No accessory particles are found by analytical ultracentrifugation (Fig. 5). In immunoelectrophoresis the virus migrates as a single band slowly towards the anode at a rate of 12.4 x 10-7 cm2 sec-1 volt -1 in 0.8% Ionagar in 0.033 M phosphate at pH 7.6.
Ultraviolet absorbance: maximum 260 nm, minimum 246 nm, with slight shoulder at 290 nm.
A260/A280: 1.25±0.02; A260/A246: 1.27±0.02, both after correction for light-scattering (Brunt & Kenten, 1971).
Particle Structure
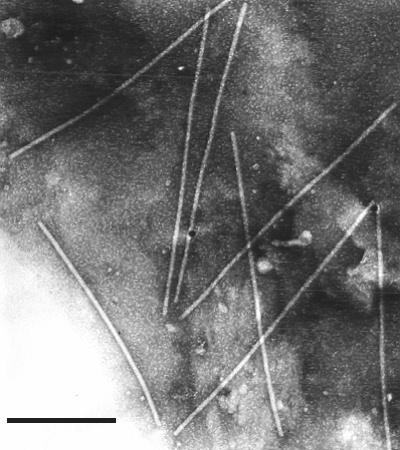
Virus preparations contain numerous flexuous filamentous particles which, in neutral phosphotungstate, measure c. 12 x 770 nm (Brunt & Kenten, 1971). After exposure to 0.05 M MgCl2, the particles become straighter and measure c. 850 nm, but they can be restored to the shorter flexuous form by removing Mg ions by dialysis against 0.02 M disodium ethylenediamine-tetraacetate at pH 7.7 (Govier & Woods, 1971). Most samples of sap from systemically infected leaves contain flexuous particles c. 12 x 750 nm (Fig. 7), but some from recently infected leaves contain either straight filaments 12 x 850 nm (Fig. 8) or a mixture of straight and filamentous particles. Particles mounted in uranyl formate show some surface structure (Fig. 6).
Particle Composition
RNA: c. 6% of the particle weight (estimated from A260/A280 ratio), probably single-stranded with molar percentages of nucleotides: G24; A23; C27; U26.
Protein: Probably a single protein species with subunits weighing c. 32,000-33,000 daltons (Brunt & Kenten, 1971).
Relations with Cells and Tissues
No amoeboid intracellular inclusions were detected in solanaceous hosts by light microscopy, but sap of infected plants contains inclusion body material (Brunt & Kenten, unpublished) similar to that induced by other members of the potato Y group (e.g. Hiebert et al., 1971).
Notes
Of the eight adequately described viruses reported to infect Capsicum frutescens and C. annuum (Martyn, 1968), only tobacco etch and potato virus Y have particles morphologically similar to those of pepper veinal mottle virus. The latter, however, is not serologically related to these two viruses, and can also be readily distinguished from them by failing to infect Solanum tuberosum, Lycopersicon esculentum and Datura stramonium, and causing only local infection in Nicotiana tabacum (Brunt & Kenten, 1971).
Pepper veinal mottle virus resembles henbane mosaic, bean yellow mosaic, and several newly recognised viruses in having particles whose length depends on their environment (Govier & Woods, 1971; Brunt & Atkey, 1971).
Figures
References list for DPV: Pepper veinal mottle virus (104)
- Brunt & Atkey, Rep. Glasshouse Crops Res. Inst. 1970: 152, 1971.
- Brunt & Kenten, Ann. appl. Biol. 69: 235, 1971.
- Govier & Woods, J. gen. Virol. 13: 127, 1971.
- Hiebert, Purcifull, Christie & Christie, Virology 43: 638, 1971.
- Kassanis & Govier, J. gen. Virol. 10: 99, 1971.
- Martyn, Phytopath. Pap. 9: 31, 1968.