Details of DPV and References
DPV NO: 106 October 1972
Family: Bromoviridae
Genus: Ilarvirus
Species: Tobacco streak virus | Acronym: TSV
Black raspberry latent virus is now known to be a distinct strain of tobacco streak virus
(Jones & Mayo, Ann. appl. Biol. 79: 297, 1975.)
Black raspberry latent virus
R. M. Lister Dept. of Botany and Plant Pathology, Purdue University, Lafayette, lndiana, USA
R. H. Converse USDA ARS Plant Science Research Division, Dept. Botany and Plant Pathology, Oregon State University, Corvallis, Oregon, USA
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by
Converse & Lister (1969,
1970).
Synonym
- New Logan-64 virus (Rev. appl. Mycol. 46: 3311n)
-
A virus with isometric particles about 26 nm in diameter, which occurs with no apparent symptoms in black raspberry (Rubus occidentalis L.) cultivars in the eastern USA, and is transmissible by sap-inoculation to a fairly wide range of herbaceous hosts. It has no known vectors, but in black raspberry is transmitted by pollen to pollinated plants and through seed.
Main Diseases
The virus may be obtained from symptomless black raspberry plants, and is not associated with any specific disease syndrome in raspberry. It has not been reintroduced into healthy raspberry plants for observation of growth or yield effects.
Geographical Distribution
In the eastern USA the virus is probably common in cultivated black raspberry though rare in red raspberry. It was obtained from Thornless Youngberry (a Rubus hybrid) collected in western Canada (R. M. Lister, unpublished), but is not reported elsewhere.
Host Range and Symptomatology
Experimental host range is fairly wide; about 30 species in 8 dicotyledonous families have been infected by sap-inoculation. Many hosts produce only mild symptoms or none.
-
Diagnostic species
- Chenopodium quinoa.
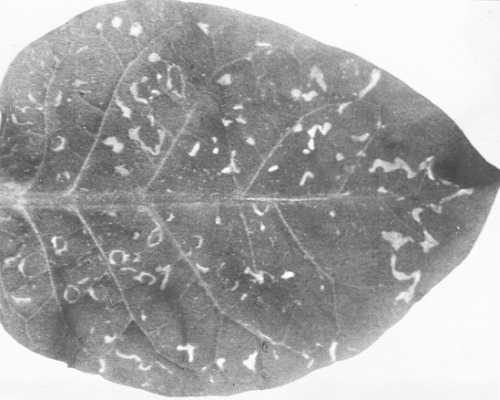
Local vein chlorosis in inoculated leaves after 2-3 days, followed by irregularly-shaped spreading necrotic and chlorotic lesions, and severe systemic chlorosis, necrosis and distortion of shoot tips (Fig. 1). Symptomless infected leaves may be produced later. - Nicotiana tabacum cvs. White Burley and Turkish. Local chlorotic and
necrotic ringspots and line patterns
(Fig. 2); virus is recoverable from symptomless
new leaves.
- Cucumis sativus cv. National Pickling. Chlorotic lesions in inoculated cotyledons, with severe systemic mottling, distortion and dwarfing (Fig. 3).
- Phaseolus vulgaris cvs. The Prince and Bountiful. No symptoms in inoculated leaves. Irregular systemic red-brown veinal necrosis, especially visible on the underside of leaves; also browning and reddening of nodes.
- Gomphrena globosa. Occasional small red rings and spots.
- Cucumis sativus cv. National Pickling. Chlorotic lesions in inoculated cotyledons, with severe systemic mottling, distortion and dwarfing (Fig. 3).
-
Propagation species
- Chenopodium quinoa
is the best source plant for bulk propagation; Cucumis sativus, though unproductive, is useful as a source of inoculum. Gomphrena globosa is suitable for maintaining cultures.Assay species
- No reliable local lesion hosts are known. Some Chenopodium spp. give
lesions sporadically:
- C. quinoa is the best systemic assay species.
Strains
No major variants have been distinguished. The isolate described in detail by Converse & Lister (1969) was from black raspberry, Rubus occidentalis, cv. New Logan, collected in the eastern USA (Converse, Lister & Cadman, 1966; Converse, 1967).
Transmission by Vectors
No aerial or soil-living vector is known. Not transmitted experimentally by Amphorophora agathonica under conditions in which this vector transmitted raspberry mosaic viruses. In black raspberry the virus is transmitted by pollen to pollinated plants.
Transmission through Seed
Experimentally seed-transmitted through about 10% of open-pollinated seed of black raspberry cultivars New Logan and Cumberland. Seed-transmission not tested in other hosts.
Transmission by Dodder
Not reported.
Serology
The virus is poorly immunogenic. Antisera obtained from rabbits given various schedules of intramuscular and intravenous injections of untreated or aldehyde-fixed virus reacted specifically only at dilutions less than 1/8.
Relationships
Lacking good antisera, testing for possible relationships between this virus and others has been based on one-way serological tests, and cross-protection tests (Converse & Lister, 1969). Serologically, the virus is unrelated to the following viruses: almond calico, arabis mosaic, cherry rugose mosaic, cucumber mosaic, prune dwarf, raspberry ringspot, sowbane mosaic, strawberry latent ringspot, sweet cherry narrow leaf, tobacco necrosis, tobacco ringspot, tomato black ring, tomato ringspot and tobacco streak. Barnett & Murant (1970) found no serological relationship to raspberry bushy dwarf virus. Cross-protection tests suggest no relationship to prunus necrotic ringspot or tobacco streak viruses.
Stability in Sap
In Chenopodium quinoa sap (1:1 w/v, in 0.1 M phosphate buffer at pH 7), thermal inactivation point is 46-49°C, dilution end-point is up to 10-4 and longevity in vitro is a few hours at 20°C or 3-5 days at 4°C. Extracts made at pH 5 have lower infectivity than those made at pH 8, but it persists longer - up to 23 days at 4°C. Freezing infected leaves or sap drastically lowers infectivity. Reducing agents only slightly increase retention of infectivity; therefore oxidation seems to be only a minor factor in affecting longevity.
Purification
The best method involves clarifying C. quinoa extracts by acidification. Extraction at pH 6 gives higher yields than extraction at pH 5 or 7, but yields are low at best (up to 0.5 mg/100 g leaf). Component ratios (see below) are influenced by the pH of extraction.
Blend infected leaves (1:1.5 w/v) with acetate (0.2 M) or phosphate (0.1 M) buffer at pH 6, containing 0.1 M sodium diethyldithiocarbamate and 0.2 M sodium thioglycollate. Squeeze through cheesecloth and adjust pH to 5 with acetic acid. Readjust to pH 6 after 1 hr and leave overnight. Centrifuge at low speed, and then subject the straw-coloured supernatant fluid to 2 cycles of differential centrifugation, resuspending the high-speed pellets in 0.1 M pH 5 acetate buffer. Concentration by precipitation with 10% polyethylene glycol (av. M. Wt 6000-7500) can be substituted for the first cycle of differential centrifugation. Further purification can be obtained by sucrose rate-zonal density gradient ultracentrifugation.
Do all steps at 4°C. Purified virus is degraded by brisk agitation.
Properties of Particles
Three nucleoprotein components occur (top, middle and bottom) with respective s20, w values of 81, 89 and 98 S at pH 5, or 78, 88 and 93 S at pH 7 (not extrapolated to infinite dilution). Only the bottom component particles appear to be infective, and the role of the others is unknown.
A260/A280: 1.6.
Particle Structure
Stained in phosphotungstate at pH 5, or after pretreatment at pH 7 with 1% glutaraldehyde (Lister & Cathro, 1967), the particles are roughly isometric, about 26 nm in diameter. Size and shape is rather variable, possibly because of distortion (Fig. 4).
Particle Composition
Details unknown.
Relations with Cells and Tissues
Details unknown: exposure of infected black raspberry plants to a constant temperature of 37°C for 16 days failed to eliminate the virus.
Notes
In the absence of good antisera and definitive symptoms in its natural host, the routine differential diagnosis of the virus is unsatisfactory. Production of systemic necrosis in C. quinoa by a virus from raspberry is not presumptive evidence for infection by black raspberry latent virus because a strain of tobacco streak virus is common in some Rubus cultivars (R. H. Converse, in press). Serological tests are needed to distinguish black raspberry latent virus from other viruses with similar herbaceous host ranges and symptomatology.
Figures
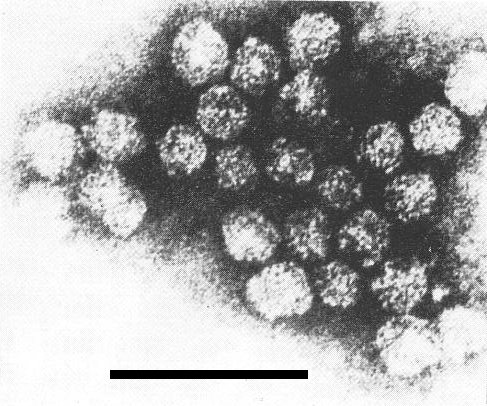
A selected field from an unfractionated virus preparation, negatively stained with phosphotungstate (pH 7) after pretreatment with glutaraldehyde. Note variations in apparent particle shape, presumably due to distortion. Some particles appear to be disintegrating. Bar represents 100 nm (photo by C. E. Bracker).
References list for DPV: Black raspberry latent virus (106)
- Barnett & Murant, Ann. appl. Biol. 65: 435, 1970.
- Converse, Lister & Cadman, Rep. Scott. hort. Res. Inst. 1965: 51, 1966.
- Converse, Phytopathology 57: 97, 1967.
- Lister & Cathro, Rep. Scott. hort. Res. Inst. 1966: 63, 1967.
- Converse & Lister, Phytopathology 59: 325, 1969.
- Converse & Lister, in Virus diseases of small fruits and grapevines, Univ. Calif Div. Agr. Sci., Berkeley: 151, 1970.