Details of DPV and References
DPV NO: 108 October 1972
Family: Secoviridae
Genus: Comovirus
Species: Bean pod mottle virus | Acronym: BPMV
Bean pod mottle virus
J. S. Semancik Department of Plant Pathology, University of Nebraska, Lincoln, Nebraska 68503, USA
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by
Zaumeyer & Thomas (1948).
Synonyms
- Pod mottle virus (J. agric. Res. 77: 81)
- Marmor valvolorum (J. agric. Res. 77: 81)
An RNA-containing virus with isometric particles about 30 nm in diameter. It infects mainly Phaseolus spp. and Glycine spp., is transmitted by beetles and readily by sap inoculation. Two kinds of nucleoprotein particles, both of which are required for infection, occur in infected plants, as well as RNA-free protein shells. Purified virus can be separated into two electrophoretic forms differing in specific infectivity.
Main Diseases
Causes severe mosaic, and mottled pods, on bush beans (Phaseolus spp.) and soybean (Glycine max) (Zaumeyer & Thomas, 1948). Locally severe on soybeans in southeastern Virginia and northeastern North Carolina, USA.
Geographical Distribution
Southern and eastern USA.
Host Range and Symptomatology
Host range limited. Only hosts known are members of the Leguminosae, principally Phaseolus vulgaris and Glycine max. Transmitted by beetles and readily by sap inoculation. Other reported hosts are Lespedeza sp., Stizolobium deeringianum and Trifolium incarnatum (Skotland, 1958).
-
Diagnostic species
- Glycine max
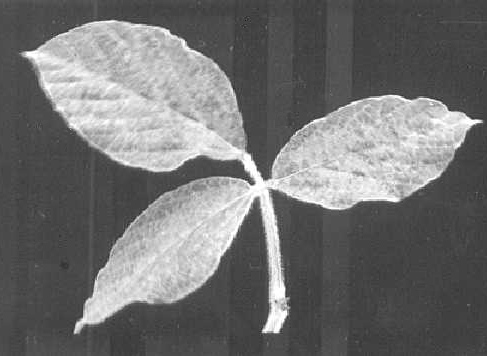
(soybean). Severe systemic mottle (Fig. 1) with mild puckering of trifoliolate leaves (Fig. 2). No stunting. Pods and seed coats mottled. - Phaseolus vulgaris cv. Tendergreen (green-podded bush snap bean).
Primary chlorotic lesions in inoculated leaves. Severe systemic mottle with
extensive chlorosis. Infected leaves are malformed but not blistered or puckered.
Pods are severely mottled, abnormally dark green, short, malformed, curled,
twisted and somewhat rough and warty; they contain abortive or abnormally developed
ovules
(Zaumeyer & Thomas, 1948).
- Phaseolus vulgaris cv. Pinto. Diffuse, reddish local lesions (Fig. 3) which appear 3-4 days after inoculation and may coalesce; sometimes vein necrosis develops in inoculated leaves. No systemic symptoms.
- Phaseolus vulgaris cv. Bountiful. Large, yellowish local lesions (Fig. 4) appearing 3-4 days after inoculation; no systemic symptoms.
- Phaseolus vulgaris cv. Pinto. Diffuse, reddish local lesions (Fig. 3) which appear 3-4 days after inoculation and may coalesce; sometimes vein necrosis develops in inoculated leaves. No systemic symptoms.
-
Propagation species
- Phaseolus vulgaris
cvs. Cherokee Wax or Black Valentine. - Glycine max cvs. Biloxi or Gibson.
-
Assay species
- Phaseolus vulgaris
cv. Pinto is suitable for local lesion assay.
Strains
The type strain was collected in South Carolina, USA (Zaumeyer & Thomas, 1948). Isolates from naturally infected soybean in North Carolina and Arkansas have not been compared with the type strain. A virus related to bean pod mottle virus and obtained from Desmodium paniculatum may be a strain (Lee & Walters, 1970).
Transmission by Vectors
Transmitted principally by the bean leaf beetle, Ceratoma trifurcata (Ross, 1963b; Walters, 1964, 1969). Virus is acquired in 2 hr, inoculated in 12 hr and retained for 2-7 days. The virus can be recovered from regurgitated food and from faeces but not from haemolymph (Slack & Fulton, 1971). Other beetle vectors reported are Diabrotica balteata, D. undecimpunctata howardii, Colaspis flavida, C. lata (Horn et al., 1970) and Epicauta vittata (Patel & Pitre, 1971).
Transmission through Seed
No evidence was obtained for seed transmission in soybean (Ross, 1963a; Skotland, 1958).
Transmission by Dodder
Not tried.
Serology
The virus is strongly immunogenic. Antisera give strong reactions in Ouchterlony double diffusion or ring precipitin tests with purified virus or with separated centrifugal and electrophoretic components, which do not differ serologically (Bancroft, 1962).
Relationships
The virus is serologically related to other members of the cowpea mosaic virus group: isolates of cowpea mosaic virus from Arkansas, Trinidad and Surinam (Shepherd, 1963, 1964; Agrawal & Maat, 1964), radish mosaic virus and squash mosaic virus (Campbell, 1964), red clover mottle virus (Gibbs, Giussani-Belli & Smith, 1968), F, (pea green mottle virus) (Valenta & Gressnerova, 1966), and a virus from Desmodium paniculatum (Lee & Walters, 1970).
Stability in Sap
In bean sap, the thermal inactivation point (10 min) is 70-75°C; dilution end-point 10-4-10-5 and longevity in vitro 62-93 days at 18°C (Zaumeyer & Thomas, 1948).
Purification
Yields of 30-40 mg virus per kg fresh leaves of soybean or bush bean can be obtained by a modification (Bancroft, 1962) of Steere’s chloroform-butanol method. Mince frozen tissue (100 g) in 3.5 ml 50% K2HPO4, express the juice and extract the fibre with 150-200 ml of 0.01 M, pH 7.0, phosphate buffer. Combine the extracts and clarify with n-butanol + chloroform. Store the aqueous phase overnight at 4°C or frozen (Semancik & Bancroft, 1964). Following one cycle of differential centrifugation, precipitate the virus by adjusting the pH to 5.0 with 10% acetic acid. Resuspend the precipitate in 0.5 M phosphate, pH 7.0, before an additional cycle of differential centrifugation. Omit the isoelectric precipitation step when studying the electrophoretic components.
Properties of Particles
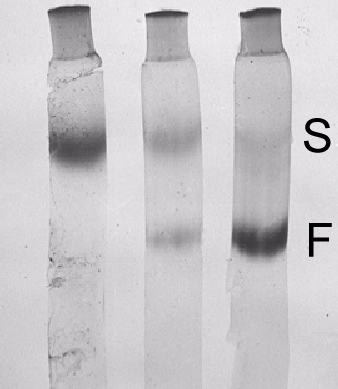
Purified virus preparations contain three centrifugal components (Fig. 6): RNA-free protein shells (T; 54 S) and two kinds of nucleoprotein particles, M (91 S) and B (112 S). Infectivity of partially separated M and B preparations is increased 7-fold when they are remixed (Wood & Bancroft, 1965). As with cowpea mosaic virus, both particle types are probably required for infection (Bruening & Agrawal, 1967). Purified virus preparations also contain two electrophoretic components (Fig. 7), fast (F) and slow (S), both infectious and containing the 54, 91 and 112 S sedimenting components (Bancroft, 1962). The S:F ratio increased with age of infection as the specific infectivity of the unfractionated preparations decreased (Gillaspie & Bancroft, 1965). Treatment with trypsin converted F particles to S particles and decreased the specific infectivity (Niblett & Semancik, 1969). There is evidence that M particles carry information that determines the serological specificity of the virus (Moore & Scott, 1971).
Sedimentation coefficients (s20,w) at infinite dilution (svedbergs): 54 (T), 91 (M) and 112 (B). Proportions of components (from Schlieren patterns) about 1:7:7.
Molecular weight: Probably about 5 x 106 (T), 6.5 x 106 (M) and 7.5 x 106.
Isoelectric point: pH 5.3 (F) and pH 4.8 (S).
Electrophoretic mobility: in 0.1 ionic strength, pH 7.0 phosphate buffer, (F) -7.49 x 10-5 and (S) -2.43 x 10-5 cm2 volt-1 sec-1 (Semancik & Bancroft, 1965). If the pH 5.0 precipitation step is included in the purification schedule the mobilities are altered to (F) -2.6 x 10-5 and (S) -0.60 x 10-5 cm2 volt-1 sec-1 (Bancroft, 1962).
Absorbance at 260 nm (1 mg/ml, 1 cm light path): 8.7 (unfractionated preparation; Gillaspie & Bancroft, 1965).
A260/A280: 1.70 (M); 1.77 (B).
Particle Structure
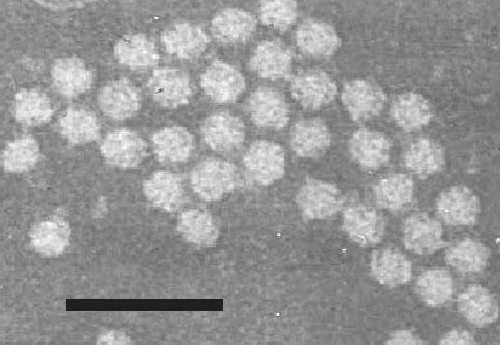
Particles are isometric, about 30 nm in diameter (Fig. 5). The 91 S (M) component is less stable than the 112 S (B) component in an alkali- chloroform medium (Semancik & Bancroft, 1965).
Particle Composition
Nucleic acid: The 91 S and 112 S components have single- stranded, linear RNA comprising respectively about 30 and 37% of the particle weight, with M. Wts of about 1.5 and 2.5 x 106. The molar nucleotide percentage for the 91 S (M) component is G19.1; A33.4; C16.7; U30.8 and for the 112 S (B) component G20.9; A31.6; C15.8; U31.6.
Protein: About 160-165 amino acid residues per subunit. A net loss of about 3-7 amino acids accompanies conversion of the fast electrophoretic form to the slow form. Numbers of residues per fast component subunit: lys 8; his 2; arg 5; asp 17; thr 12; ser 16; glu 14; pro 8; gly 16; ala 11; val 12; met 6; ile 10; leu 14; try 2; phe 9.
Relations with Cells and Tissues
Particles were observed within tubules embedded in the cell wall of the systemic host, Cherokee Wax bean, and between the plasmalemma and cell wall in the local lesion host, Pinto bean (Kim & Fulton, 1971).
Notes
The virus can be distinguished from southern bean mosaic virus by its lower thermal inactivation point and multiple component distribution on sedimentation. Also, Vigna sinensis (cowpea), a useful propagation and local lesion host for southern bean mosaic virus, is not susceptible to bean pod mottle virus. Other members of the cowpea mosaic virus group of beetle-transmitted viruses, such as cowpea mosaic, squash mosaic, radish mosaic, red clover mottle, and possibly broad bean stain viruses, should be distinguished serologically.
Figures
References list for DPV: Bean pod mottle virus (108)
- Agrawal & Maat, Nature, Lond. 202: 674, 1964.
- Bancroft, Virology 16: 419, 1962.
- Bruening & Agrawal, Virology 32: 306, 1967.
- Campbell, Phytopathology 54: 1418, 1964.
- Gibbs, Giussani-Belli & Smith, Ann. appl. Biol. 61: 99, 1968.
- Gillaspie & Bancroft, Phytopathology 55: 906, 1965.
- Horn, Newsom, Carver & Jenson, La Agric. 13: 12, 1970.
- Kim & Fulton, Virology 43: 329, 1971.
- Lee & Walters, Phytopathology 60: 585, 1970.
- Moore & Scott, Phytopathology 61: 831, 1971.
- Niblett & Semancik, Virology 38: 685, 1969.
- Patel & Pitre, Pl. Dis. Reptr 55: 628, 1971.
- Ross, Phytopathology 53: 887, 1963a.
- Ross, Pl. Dis. Reptr 47: 1049, 1963b.
- Semancik & Bancroft, Virology 22: 33, 1964.
- Semancik & Bancroft, Virology 27: 476, 1965.
- Shepherd, Phytopathology 53: 865, 1963.
- Shepherd, Phytopathology 54: 466, 1964.
- Skotland, Pl. Dis. Reptr 42: 1155, 1958.
- Slack & Fulton, Virology 43: 728, 1971.
- Valenta & Gressnerova, Acta virol., Prague 10: 182,1966.
- Walters, Phytopathology 54: 240, 1964.
- Walters, Adv. Virus Res. 15: 339, 1969.
- Wood & Bancroft, Virology 27: 94, 1965.
- Zaumeyer & Thomas, J. agric. Res. 77: 81, 1948.