Details of DPV and References
DPV NO: 121 July 1973
Family: Secoviridae
Genus: Comovirus
Species: Radish mosaic virus | Acronym: RaMV
Radish mosaic virus
R. N. Campbell Department of Plant Pathology, University of California, Davis, California 95616, USA
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by
Tompkins (1939) and rediscovered by
Campbell (1964).
Selected synonyms
- Marmor raphani
(Rev. appl. Mycol. 28: 514) - Raphanusvirus maculans (Rev. appl. Mycol. 38: 677)
- Radish enation mosaic virus (Rev. appl. Mycol. 47: 2309)
-
An isometric RNA virus c. 30 nm in diameter with multiple sedimenting and electrophoretic components, transmissible by beetles and by inoculation of sap. It occurs naturally only in cruciferous plants but can be inoculated to a few non- crucifers. Widely distributed.
Main Diseases
Causes mosaic of turnip and radish. Symptoms commonly include ringspots and crinkling of leaves; radish plants may develop enations. In California (Campbell, 1964) and Japan (Tochihara, 1968) the virus has been isolated only occasionally; in Yugoslavia it is the most common virus in turnip (Stefanac & Mamula, 1972).
Geographical Distribution
Known from USA (California), Japan and Europe.
Host Range and Symptomatology
Infects most crucifers, causing mosaic, ringspots (chlorotic or necrotic), veinal necrosis, leaf crinkling and, occasionally, systemic necrosis. Some hosts, e.g. Tendergreen mustard, are heterozygous for susceptibility; others show varietal differences in susceptibility, e.g. Purple Top White Globe turnip is resistant, Early White Flat Dutch and Shogoin turnips are susceptible. Additional hosts in the Solanaceae, Chenopodiaceae and Cucurbitaceae give local lesion reactions but may remain symptomless in the summer in California.
-
Diagnostic species
- Brassica campestris
Perviridis group (Tendergreen mustard). Susceptible plants develop chlorotic or necrotic lesions (Fig. 1), followed by systemic mosaic with chlorotic ringspots. - Brassica oleracea. Local and systemic chlorotic ring patterns
(Fig. 3)
or symptomless infection, depending on the isolate.
- Raphanus sativus (radish) cvs. White Icicle, Chinese White Winter. Systemic mottle usually with ringspots; enations develop later (Fig. 2, Fig. 5).
- Chenopodium amaranticolor. Necrotic local lesions (not in summer in California). Not systemic.
- Nicotiana tabacum (tobacco) cv. Havana 425. No symptoms.
- N. tabacum cvs. Turkish and Samsun. Chlorotic local lesions or necrotic rings (Fig. 4). Not systemic.
- Raphanus sativus (radish) cvs. White Icicle, Chinese White Winter. Systemic mottle usually with ringspots; enations develop later (Fig. 2, Fig. 5).
-
Propagation species
- B. campestris
Perviridis group, B. campestris Chinensis group (Chinese mustard or Bock Toy), and B. campestris Rapifera group (turnip) have been used to maintain cultures and as sources of virus for purification.Assay species
- C. amaranticolor
is a satisfactory local lesion host but may not form lesions during summer.
Strains
All isolates are similar in host range and reaction. The neo-type strain (Campbell, 1964) found in California, USA, is deposited with the American Type Culture Collection as PV-138.
Transmission by Vectors
Transmission has been achieved with the beetles Phyllotreta spp., Epitrix hirtipennis and Diabrotica undecimpunctata (Campbell & Colt, 1967; Tochihara, 1968; Stefanac & Mamula, 1972; Walters, 1969). Virus-vector relations have not been studied; only adult vectors have been used.
Transmission through Seed
No seed transmission was obtained in radish (Campbell & Colt, 1967; Tochihara, 1968).
Transmission by Dodder
No information.
Serology
The virus is a good immunogen. Gel-diffusion tests with crude sap of systemically infected crucifers give a good reaction with a single line of precipitation. Immuno-electrophoresis gives two components (Campbell, unpublished, Fig. 7).
Relationships
Isolates from California and Japan were serologically identical (Campbell & Tochihara, 1969) whereas the HZ strain from Yugoslavia was closely related to but distinguishable from the Californian isolate (Stefenac & Mamula, 1972). The Californian strain was distantly serologically related to cowpea mosaic, bean pod mottle and squash mosaic viruses (all members of the cowpea mosaic virus (comovirus) group) but did not react with antisera to eleven other polyhedral viruses including turnip crinkle, turnip yellow mosaic and turnip rosette viruses (Campbell, 1964 and unpublished).
Stability in Sap
Thermal inactivation point is between 65 and 70°C, longevity at room temperature is from 2 to 3 weeks, dilution end-point is about 1/15,000. Similar results were obtained when Tendergreen mustard, turnip, or radish were the source plants (Campbell, 1964; Tochihara, 1968; Stefanac & Mamula, 1972).
Purification
1. Campbell (1964). Grind leaves at room temperature in 0.5 M KH2PO4-Na2HPO4 (pH 7.6) buffer, squeeze through cheesecloth, add n-butanol to 8% of total volume and stir for 1-2 h. Clarify, then chill to precipitate phosphate. Concentrate virus from supernatant fluid by 2 cycles of differential centrifugation. Yields up to 15 mg/100 g of leaves; virtually free of host material.
2. Stefanac & Mamula (1972) used Steere’s chloroform technique.
Properties of Particles
Purified virus preparations contain three centrifugal components (Fig. 6): top (T), found in trace amounts, is probably free of RNA by analogy with cowpea mosaic and bean pod mottle viruses; middle (M) and bottom (B) contain RNA. Infectivity is greatest when M and B are mixed (Kodama, 1971). Purified preparations also contain two electrophoretic components (Fig. 7), fast (F) and slow (S), which have distinct isoelectric points; each contains at least the M and B centrifugal components (Campbell, unpublished).
Sedimentation coefficients (s20) in 0.05 M phosphate buffer with 0.1 M NaCl at infinite dilution (svedbergs): about 57 (T), 97 (M) and 116 (B) (Campbell, unpublished; Kodama, 1971). Ratio of M:B (from schlieren patterns) varies from 2:1 to 4:1.
Diffusion coefficient: approx. 1.30 x 10-7 cm2/sec.
Electrophoretic mobility: two components (F and S) detectable in cellulose acetate electrophoresis, immuno-electrophoresis, and electrophoresis in 2.4% polyacrylamide gels using 0.01 ionic strength phosphate buffer (pH 6.8) (Campbell, unpublished).
Isoelectric points: pI = 4.3 (approx) (F); pI = 5.3 (approx) (S) by ampholine isoelectric focusing.
Absorbance at 260 nm (1 mg/ml, 1 cm light path): 9 (M), 11 (B).
A260/A280: 1.65 (M), 1.78 (B).
Particle Structure
Particles are isometric, about 30 nm in diameter (Campbell, 1964; Tochihara, 1968). In negatively stained preparations some particles appear hollow (Fig. 8).
Particle Composition
Nucleic acid: M and B components contain RNA comprising about 26 and 35%
of the particle, respectively. RNA species extracted from non-fractionated
preparations have M. Wt about 1.3 and 2.2 x 106 (Campbell, unpublished).
The molar percentages are about G22; A29; C19; U30 for M, and G25; A30; C18; U27
for B (Campbell, unpublished).
Protein: No information.
Relations with Cells and Tissues
No tissue restriction known. Aggregates of virus occur in the cytoplasm and in vacuoles, especially at the interface between them; often particles occur in multiple rows within membrane-bounded tube-like structures that are regarded as tonoplastic channels (Honda & Matsui, 1972; Hooper, Spink & Myers, 1972). Large, vesiculated inclusion bodies containing aggregates of virus particles are described in turnip epidermal cells (Stefanac & Ljubesic, 1971).
Notes
Radish mosaic, turnip yellow mosaic, turnip crinkle and turnip rosette viruses are all 30 nm diameter, isometric viruses isolated from Cruciferae. Host reaction and host range cannot reliably distinguish among them; both radish mosaic and turnip crinkle viruses infect hosts outside the Cruciferae. Serology is the most rapid, reliable diagnostic procedure and can be employed with crude sap expressed from infected leaves. Biophysical characteristics of radish mosaic virus differ from those of the other three viruses. The unusual properties of the radish stunt virus of Isiyama & Misawa (1943) are thought to be caused by a complex of radish mosaic and turnip mosaic viruses (Tochihara, 1968; Campbell & Tochihara, 1969).
Figures
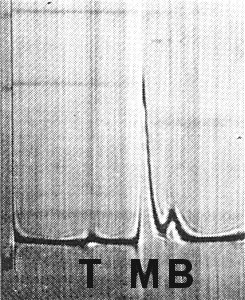
Schlieren pattern of partially purified virus preparation sedimenting left to right, after 8 min at 35,600 rpm.
References list for DPV: Radish mosaic virus (121)
- Campbell, Phytopathology 54: 1418, 1964.
- Campbell & Colt, Phytopathology 57: 502, 1967.
- Campbell & Tochihara, Phytopathology 59: 1756, 1969.
- Honda & Matsui, Phytopathology 62: 448, 1972.
- Hooper, Spink & Myers, Virology 47: 833, 1972.
- Isiyama & Misawa, Ann. phytopath. Soc. Japan 12: 116, 1943.
- Kodama, Kagaku to Seibutu 9: 155, 1971.
- Stefanac & Ljubesic, J. gen. Virol. 13: 51, 1971.
- Stefanac & Mamula, Ann. appl. Biol. 69: 229, 1972.
- Tochihara, Ann. phytopath. Soc. Japan 34: 129, 1968.
- Tompkins, J. agric. Res. 58: 119, 1939.
- Walters, Adv. Virus Res. 15: 339, 1969.






