Details of DPV and References
DPV NO: 125 July 1973
Family: Unallocated ssRNA+ viruses
Genus: Sobemovirus
Species: Turnip rosette virus | Acronym: TRoV
Turnip rosette virus
M. Hollings Glasshouse Crops Research Institute, Littlehampton, Sussex, England
Olwen M. Stone Glasshouse Crops Research Institute, Littlehampton, Sussex, England
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
Described by Blencowe & Broadbent (1957) and Broadbent & Heathcote (1958).
A virus with isometric particles about 28 nm in diameter. Found rarely in turnip (Brassica rapa) and swede (B. napus) crops in Scotland and transmitted by inoculation of sap and possibly by flea-beetles. It infects very few hosts outside the Cruciferae.
Main Diseases
In turnip the virus induces necrosis of petioles and veins, with severe dwarfing, leaf twisting and rosetting. In swede it causes vein-banding, rosetting and stunting (Broadbent & Heathcote, 1958).
Geographical Distribution
Recorded only twice, both times in Scotland.
Host Range and Symptomatology
Known hosts are restricted to 20 species in the Cruciferae, Compositae, Resedaceae and Solanaceae.
-
Diagnostic species
- Brassica pekinensis
(Pe-tsai, Chinese cabbage). Local necrotic and occasional chlorotic spots in 6-10 days (Fig. 1). The rarity of systemic infection (veinal necrosis, leaf distortion and crinkle; Fig. 2) is of diagnostic value. - B. napus (swede). Local chlorotic spots; systemic vein-clearing, followed
by necrosis of
the petioles and leaf veins, with leaf twisting and severe rosetting.
Infected plants are commonly
killed at c. 20°C, but show milder symptoms at c. 10°C.
- B. rapa (turnip). Local chlorotic or necrotic lesions; systemic vein-clearing, followed by necrosis of the petioles and leaf veins, dwarfing, leaf twisting and rosetting (Fig. 3, Fig. 4). Symptoms are much more severe at 20°C than at 10°C.
- The virus infects inoculated leaves of Nicotiana clevelandii and N. bigelovii without causing definite symptoms, and does not infect N. tabacum.
Propagation species
- Brassica pekinensis
is a good source of virus for purification but, because systemic infection is unusual, it is less satisfactory for maintaining cultures. The virus can be maintained in B. rapa.Assay species
- Brassica pekinensis
gives satisfactory local lesions. - B. rapa (turnip). Local chlorotic or necrotic lesions; systemic vein-clearing, followed by necrosis of the petioles and leaf veins, dwarfing, leaf twisting and rosetting (Fig. 3, Fig. 4). Symptoms are much more severe at 20°C than at 10°C.
Strains
Only two isolates have been recorded (Broadbent & Heathcote, 1958; Lister, 1958); there is no evidence for any difference between them.
Transmission by Vectors
Probably transmitted by biting insects (Martini, 1958), but no details have been published.
Transmission through Seed
No information.
Transmission by Dodder
Not recorded.
Serology
The virus is a good immunogen; rabbits immunized by one intravenous plus two intramuscular injections (with adjuvant) gave antisera with specific titres in precipitin tube tests of 1/8192 (Hollings & Stone, 1969). The precipitates are granular (somatic). In gel-diffusion tests, a single reaction line is formed; good reactions are obtained with crude infective sap.
Relationships
No serological relationships were found to any of twenty-seven other isometric viruses (Hollings & Stone, 1969).
Stability in Sap
In Brassica pekinensis sap, the thermal inactivation point is 85-90°C, rarely 95°C, the dilution end-point is 10-5-10-6 and infectivity survives at least 30 days at laboratory temperature. Lyophilized sap containing 7% dextrose + 7% peptone and stored under vacuum at laboratory temperature retained infectivity for at least 5 years.
Purification
The following method gives good yields (Hollings & Stone, 1969): preparations from Brassica pekinensis may yield 10-14 mg virus per kg leaf tissue. Harvest inoculated leaves of B. pekinensis 10-15 days after inoculation. Homogenize leaves at room temperature with 0.05 M phosphate buffer (pH 7.6) containing 0.1% thioglycollic acid using 1.5 ml buffer/g leaf. Express through cheesecloth and add 9.3 ml n-butanol to every 100 ml extract. Shake the extract for 5 min, and store it at 2°C for 12-48 h. If this step is omitted, most of the virus may be lost. Concentrate the virus by one or more cycles of differential centrifugation. Purify further by density gradient centrifugation; dialyse the virus-containing fractions against 0.03 M phosphate buffer + 0.5% NaCl for 18 h before pelleting the virus.
Properties of Particles
Sedimentation coefficient (s20,w) at infinite dilution: 112 S.
In immunoelectrophoresis at c. 4°C, using 0.9% Ionagar in 0.03 M phosphate buffer pH 7.6, the virus migrates as a single antigenic component towards the anode at a rate of 2.40 x 10-6 cm2 sec-1 volt-1.
A260/A280: 1.56.
Amax(260)/Amin(241): 1.30.
Particle Structure
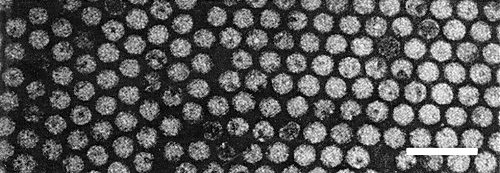
Particles are isometric, about 28 nm in diameter in phosphotungstate (Fig. 5).
Particle Composition
RNA: Molar percentages of nucleotides: G25; A26; C22; U27.
Protein: Particles contain a protein of M. Wt 27,700 (M. Hollings, O. M. Stone & R. Barton, unpublished).
Relations with Cells and Tissues
All tissues are infected; no inclusion bodies have been reported.
Notes
Several viruses induce similar symptoms in cruciferous crops. Turnip rosette can be distinguished serologically from the isometric viruses turnip crinkle, turnip yellow mosaic and radish mosaic, and unlike the last two has only one sedimenting component. The following are also useful distinguishing features. Turnip crinkle virus sediments at 129 S and induces local lesions in Chenopodium amaranticolor, C. quinoa, Tetragonia expansa and Datura stramonium. Turnip yellow mosaic and turnip crinkle viruses cause chlorotic local lesions in Brassica pekinensis. Radish mosaic virus induces necrotic local lesions in B. pekinensis, Nicotiana clevelandii and N. tabacum and has a thermal inactivation point of 68-70°C.
Another virus common in cruciferous crops, turnip mosaic, has filamentous particles, is aphid-borne, and causes severe systemic mottle and crinkling in Nicotiana clevelandii.
Figures
References list for DPV: Turnip rosette virus (125)
- Blencowe & Broadbent, Rep. Rothamsted exp. Stn 1956: 105, 1957.
- Broadbent & Heathcote, Ann. appl. Biol. 46: 585, 1958.
- Hollings & Stone, Zentbl. Bakt. ParasitKde, Abt II 123: 237, 1969.
- Lister, Pl. Path. 7: 144, 1958.
- Martini, Proc. 3rd conf. Potato Virus Diseases, Lisse-Wageningen, 1957: 106, 1958.