Details of DPV and References
DPV NO: 132 July 1974
Family: Secoviridae
Genus: Nepovirus
Species: Chicory yellow mottle virus | Acronym: ChYMV
Chicory yellow mottle virus
A. Quacquarelli Istituto di Patologia Vegetale, Università di Bari, 70126 Bari, Italy
G. P. Martelli Istituto di Patologia Vegetale, Università di Bari, 70126 Bari, Italy
C. Vovlas Istituto di Patologia Vegetale, Università di Bari, 70126 Bari, Italy
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by Vovlas, Martelli & Quacquarelli (1971) and Quacquarelli
et al. (1972c).
- A virus with angular isometric RNA-containing particles c. 30 nm in diameter, which sediment as several components when centrifuged. It is readily transmitted by inoculation of sap to a moderate range of hosts and is seedborne, but no specific vector has been found.
Main Diseases
Causes bright yellow mottle, ringspot and line pattern in the leaves of chicory (Cichorium intybus) (Fig. 1) (Vovlas et al., 1971) and obvious foliar alterations in parsley (Petroselinum crispum) (Fig. 2) (Avgelis & Quacquarelli, 1974a).
Geographical Distribution
Reported from Southern Italy.
Host Range and Symptomatology
In nature, found only in chicory and parsley. Transmitted experimentally to 32 species in 12 dicotyledonous families (Vovlas et al., 1971; Avgelis & Quacquarelli. 1974a). Several Nicotiana species are infected symptomlessly. Readily transmitted by inoculation of sap.
- A virus with angular isometric RNA-containing particles c. 30 nm in diameter, which sediment as several components when centrifuged. It is readily transmitted by inoculation of sap to a moderate range of hosts and is seedborne, but no specific vector has been found.
- Diagnostic species
- Phaseolus vulgaris (French bean). Erratic yellowish local lesions
develop in inoculated leaves. Systemic symptoms consist of chlorotic mottle,
and yellow spots and blotches (Fig. 3).
- Cucurbita pepo (squash). Occasional yellow local lesions followed by a conspicuous systemic mosaic, ringspots, deformation of the leaf blades, and small necrotic areas.
- Cucurbita pepo (squash). Occasional yellow local lesions followed by a conspicuous systemic mosaic, ringspots, deformation of the leaf blades, and small necrotic areas.
- Propagation species
- Cucurbita pepo is a good source of virus for purification.
- Assay species
- No reliable local lesion host known. Some strains may be assayed by recording the proportion of Chenopodium quinoa plants becoming infected.
- calculated according to Reichmann’s (1965) formula: 14% in B1; 22% in B2; 25 to 37% in B3; 39% in B4; 43% in B5;
- calculated from buoyant densities: 6 to 38% in isopycnic components B1 to B3; 40% in B4; and 41.5 to 43% in isopycnic components of B5.
- Avgelis & Quacquarelli, Phytopath. Mediterranea 13: 97, 1974a.
- Avgelis & Quacquarelli, Phytopath. Mediterranea 13: 160, 1974b.
- Dunn & Hitchborn, Virology 25: 171, 1965.
- Hull, Rees & Short, Virology 37: 404, 1969.
- Kaper & Alting Siberg, Virology 38: 407, 1969.
- Mink, CMI/AAB Descriptions of Plant Viruses 92, 4 pp., 1972.
- Quacquarelli, Piazzolla & Vovlas, J. gen. Virol. 17: 147, 1972a.
- Quacquarelli, Piazzolla & Vovlas, Phytopath. Mediterranea 11: 207, 1972b.
- Quacquarelli, Vovlas, Piazzolla, Russo & Martelli, Phytopath. Mediterranea 11: 180, 1972c.
- Quacquarelli, Piazzolla, Vovlas & Martelli, Mikrobiologija 10: 15, 1973.
- Reichmann, Virology 25: 166, 1965.
- Schneider, Hull & Markham, Virology 47: 320, 1972.
- Vovlas, Phytopath. Mediterranea 12: 102. 1973.
- Vovlas, Martelli & Quacquarelli, Phytopath. Mediterranea 10: 244, 1971.
Strains
Two major variants are described:
Type strain (Vovlas et al., 1971). Causes chlorotic local lesions and a characteristic wilting in Chenopodium quinoa (Fig. 6). Plants at the 6-8 leaf stage wither and die in less than a week. Older plants also wilt but may recover. Gomphrena globosa reacts with whitish red-rimmed local lesions and no systemic infection; Cucumis sativus develops yellowish local lesions and a mosaic mottle. Parsley is not a host. A minor variant of the type strain induces obvious concentric ringspots in inoculated leaves of Nicotiana glutinosa (C. Vovlas, unpublished data).
Parsley strain (Parsley carrot-leaf virus) (Avgelis & Quacquarelli, 1974a). Induces chlorotic local lesions and systemic mottle but no wilting in C. quinoa, necrotic target-like local spots, systemic mottle and necrosis in G. globosa, and yellowish local rings, systemic mosaic and enations in C. sativus. Chicory is not a host.
Transmission by Vectors
No vector known. Attempts to transmit the major strains using the aphid Myzus persicae were unsuccessful (Avgelis & Quacquarelli, 1974a).
Transmission through Seed
The type strain was transmitted to a small proportion of chicory seeds (Vovlas, 1973). No seed transmission of the parsley strain was found in several species (Avgelis & Quacquarelli, 1974a).
Transmission by Dodder
No information.
Serology
Empty protein shells (top centrifugal component) are moderately immunogenic, yielding antisera with precipitin titres no higher than 1/128. Nucleoprotein fractions (bottom centrifugal component) are good immunogens, producing antisera with titres up to 1/1024 following intramuscular and intravenous injections of rabbits (Vovlas et al., 1971; Quacquarelli et al., 1972c). Top and bottom components are serologically indistinguishable. Tube-precipitin (granular precipitates) or gel-diffusion tests (single precipitin bands) can be used for comparing strains (Quacquarelli et al., 1972c; Avgelis & Quacquarelli, 1974b).
Relationships
The chicory and parsley strains share most but not all antigenic determinants (Avgelis & Quacquarelli, 1974a, 1974b); no serological differences occur among minor variants of the same strain. No relationship was detected to 23 viruses with isometric particles including several members of the nepovirus, cucumovirus, tymovirus, comovirus and tombusvirus groups (Vovlas et al., 1971).
Stability in Sap
All strains behave similarly, irrespective of the assay host used. In expressed sap, infectivity is lost after dilution to 10-3-10-5, heating for 10 min at 53-56°C or storing for 3-4 days at 22°C (Vovlas et al., 1971; Avgelis & Quacquarelli, 1974a).
Purification
Satisfactory purification is achieved by clarification of expressed sap with 7% (v:v) of magnesium-activated bentonite (Dunn & Hitchborn, 1965) followed by cycles of low- and high-speed centrifugation and sucrose density gradient centrifugation (Vovlas et al., 1971; Avgelis & Quacquarelli, 1974a). Virus yields range from 5 to 30 mg/100 g of infected squash tissue. Spontaneous crystallization occurs at 4°C in purified virus preparations of the type strain (Fig. 5) (Quacquarelli et al., 1972c).
Properties of Particles
Purified virus preparations contain protein shells without RNA (T) and nucleoproteins containing different amounts of RNA (B). Sucrose density gradient and analytical centrifugation resolve up to five classes of particles in component B (B1-B5; Fig. 4) (Quacquarelli et al., 1972c). Equilibrium centrifugation in CsCl reveals that these components are themselves heterogeneous because, in addition to component T, there are at least 16 classes of particle in component B (Fig. 9). Most of the heterogeneity is in component B3 (Quacquarelli et al., 1973; A. Quacquarelli & A. Avgelis, unpublished data). The relative proportions of the centrifugal components varies with the virus strain, host and temperature (Fig. 4) (A. Quacquarelli & A. Avgelis, unpublished data). Infectivity is associated with the fast-sedimenting nucleoprotein fractions (B4 and B3). In infected tissues or purified suspensions, virus particles dissociate into protein shells and infective RNA when frozen at -25°C and thawed (Quacquarelli, Piazzolla & Vovlas, 1972a).
Sedimentation coefficients (s20,w) in 0.02 M phosphate buffer with 0.1 M NaCl (svedbergs): 50 (T), 66 (B1), 78 (B2), 84 to 109 (B3), 117 (B4) and 126 (B5) (Quacquarelli et al., 1973; A. Quacquarelli & A. Avgelis, unpublished data).
Buoyant densities in CsCl at 25°C: 1.279 (T), 1.298 to 1.464 (B1 to B3, split into 12 separate isopycnic components), 1.481 (B4), 1.487 to 1.505 (B3, split into 3 isopycnic components) (Quacquarelli et al., 1973).
M. Wt of T component particles is c. 3 x 106 daltons (A. Quacquarelli & P. Piazzolla, unpublished data).
Electrophoretic behaviour: The virus does not move from the origin when electrophoresed in 2.4% polyacrylamide gels or in immuno-electrophoresis, irrespective of the agar concentration or the type and pH of buffer. In zone electrophoresis it migrates to the anode in a single band with a R F value of 0.10 (Quacquarelli et al., 1972c; Avgelis & Quacquarelli, 1974b).
Absorbance at 260 nm (1 mg/ml, 1 cm light path): 9.5 (type strain, unfractionated virus).
Particle Structure
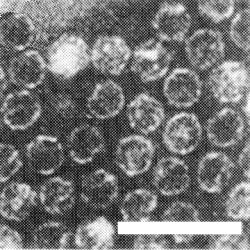
Particles are isometric, c. 30 nm in diameter with angular outlines (Fig. 7). T particles are penetrated by negative stain (Fig. 8). Some of the B particles are partially penetrated by neutral sodium phosphotungstate.
Particle Composition
Nucleic acid: single-stranded RNA, comprising the following percentages of the particle weight:
Infective RNA is easily obtained from purified virus preparations in 0.02 M (Na-K) phosphate buffer by freezing for 10 min at -25°C and thawing (Quacquarelli et al., 1972a, 1972b) or by heating at 71°C for 90 sec (A. Quacquarelli, unpublished data). Only preparations containing the 31 and 35 S RNA species are infective but it is not known whether both species are necessary for infection (Quacquarelli et al., 1973).
Protein: No information.
Relations with Cells and Tissues
Electron microscopy revealed abnormalities of the fine structure of infected squash cells but virus particles could not be identified with certainty (G. P. Martelli & M. A. Castellano, unpublished data).
Notes
The virus, owing to the distinctive symptoms induced in chicory, is easily distinguished from other viruses infecting this host in nature. The dissociation of its protein and RNA moieties in intact form, when frozen and thawed, is not known to occur with any other isometric plant virus. Turnip yellow mosaic (Kaper & Alting Siberg, 1969), peanut stunt (Western strain) (Mink, 1972), grapevine chrome mosaic, radish mosaic and artichoke Italian latent viruses (A. Quacquarelli, unpublished data) also release intact RNA when frozen but their protein shells disintegrate. In its unusual centrifugal complexity, chicory yellow mottle virus resembles preparations of tobacco ringspot virus containing satellite RNA (Schneider, Hull & Markham, 1972). However, the two viruses are not serologically related nor is there, at the moment, sufficient ground for ascribing chicory yellow mottle virus to the nepoviruses.
Figures

Ultraviolet absorption patterns of preparations of (above) parsley strain and (below) type strain after sucrose density gradient centrifugation, both purified from squash. Note the difference in the type and relative amounts of B components.

Virus particles from a purified preparation of component B, mounted in phosphotungstate. Bar represents 50 nm.

Schlieren diagram of equilibrium sedimentation analyses in CsCl of a purified virus preparation: (a) initial density 1.3029, showing density components T and 1-3; (b) initial density 1.3622, showing density components 3-7; (c) initial density 1.4154, showing density components 6-12; (d) initial density 1.4498, showing density components 9-15; (e) initial density 1.5117, showing density components 14-16.