Details of DPV and References
DPV NO: 140 July 1974
Family: Betaflexiviridae
Genus: Carlavirus
Species: Cowpea mild mottle virus | Acronym: CPMMV
Cowpea mild mottle virus
A. A. Brunt Glasshouse Crops Research Institute, Littlehampton, Sussex, England
R. H. Kenten Cocoa Research Institute, Tafo, Ghana
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
Described by Brunt & Kenten (1973).
A virus with filamentous particles c. 650 nm long. Seed-borne in some leguminous hosts and readily sap-transmissible to species in several families. No known vector. Common in the Eastern Region of Ghana.
Main Diseases
Found naturally only in cowpea (Vigna unguiculata) in which it usually causes mild leaf mottling but occasionally severe systemic chlorosis and necrosis (Fig. 1).
Geographical Distribution
Common in the Eastern Region of Ghana.
Host Range and Symptomatology
Infected 11 of 17 members of the Papilionaceae tested and 10 of 51 other species within 5 of 19 families (Brunt & Kenten, 1973).
- Diagnostic species
- Arachis hypogaea
(groundnut). Few necrotic lesions, chlorotic rings or line patterns in inoculated leaves, soon followed by systemic leaf chlorosis, leaf rolling and some veinal necrosis. Infected plants are severely stunted. - Beta vulgaris (beetroot) cv. Detroit. Fawn necrotic local lesions
(Fig. 4). No systemic infection.
- Cajanus cajan (pigeon pea). Conspicuous systemic leaf chlorosis and distortion within 14-28 days; plants are severely stunted.
- Glycine max (soybean) cv. Chippewa. Conspicuous systemic leaf chlorosis within 14-21 days (Fig. 2), occasionally followed by apical necrosis.
- Phaseolus vulgaris (French bean) cv. The Prince. Conspicuous chlorotic spotting of some systemically infected leaves.
- Nicotiana clevelandii. Inconspicuous systemic leaf chlorosis.
- Theobroma cacao (cocoa) cv. West African Amelonado. Systemic infection in about half of the seedlings grown from inoculated cocoa beans; immature leaves develop red vein-banding symptoms but mature leaves have chlorosis along secondary and tertiary veins (Fig. 5).
- Cajanus cajan (pigeon pea). Conspicuous systemic leaf chlorosis and distortion within 14-28 days; plants are severely stunted.
- Propagation species
- Glycine max
and Nicotiana clevelandii are suitable for maintaining cultures and as sources of virus for purification.- Assay species
- Chenopodium quinoa
is a useful assay host; inoculated leaves produce numerous chlorotic or necrotic lesions within 12 days (Fig. 3).
Strains
None reported.
Transmission by Vectors
Although serologically related to carnation latent virus, cowpea mild mottle virus was not transmitted in either the persistent or non-persistent manner by 12 aphid species including Acyrthosiphon pisum, Aphis craccivora, Aphis fabae and Myzus persicae. In experiments to investigate whether transmission is dependent upon a ‘helper’ virus (Kassanis & Govier, 1971a, 1971b), cowpea mild mottle virus was not transmitted by Myzus persicae first fed either on pepper plants infected with potato Y or pepper veinal mottle viruses or on Dianthus barbatus plants infected with carnation latent virus.
Transmission through Seed
Seed-borne (2-90%) in Vigna unguiculata, Glycine max and Phaseolus vulgaris but not in Nicotiana clevelandii (Brunt & Kenten, 1973).
Transmission by Dodder
No information.
Serology
Antisera with titres of 1/2048 are readily prepared, and react with homologous virus preparations to produce flagellar precipitates in precipitin tube tests.
Relationships
Serologically distantly related to carnation latent virus, but apparently unrelated to the following carlaviruses: pea streak, red clover vein mosaic, cactus 2, chrysanthemum B, passiflora latent, potato M, potato S, narcissus latent, white bryony mosaic and a virus from elderberry (Brunt & Kenten, 1973).
Stability in Sap
Sap from systemically infected Glycine max was infective to Chenopodium quinoa after dilution to 10-3 but not 10-4, after 10 min at 65°C but not 70°C, and after at least 8 days at 20°C or 20 days at 2°C (Brunt & Kenten, 1973). Lyophilized sap remains infective for at least 4 years.
Purification
Glycine max and Nicotiana clevelandii are the best sources of virus for purification. Losses of virus due to irreversible aggregation of particles are minimized by the use of low molarity alkaline buffer. The following procedure yields 2-5 mg virus/kg of leaf tissue (Brunt & Kenten, 1973). Freeze infected leaves at -20°C, then grind in 0.02 M borate at pH 9.5 (2 ml/g tissue), express the fluid through cotton cloth and centrifuge for 15 min at 10,000 g. Shake the supernatant fluid for 5 min with 0.5 vol. chloroform and, after 1 h at 2°C, centrifuge the mixture for 15 min at 10,000 g. Sediment the virus from the aqueous phase by centrifugation for 1 h at 75,000 g. resuspend the pellets in 0.01 M borate at pH 9.5 and then repeat the cycle of differential centrifugation. Resuspend the virus in 0.01 M borate, freeze it for c. 18 h, thaw and clarify by low speed centrifugation. Further purification may be achieved by centrifugation through 10-40% linear sucrose density gradients for 2-5 h at 45,000 g.
Properties of Particles
Virus preparations contain a single sedimenting component with s°20,w of 165 ± 4 S.
A260/A280: 1.14; A260/A246: 1.21 (both after correction for light-scattering).
Particle Structure
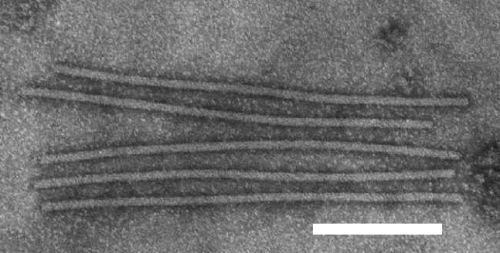
The virus has straight or slightly flexuous filamentous particles c. 13 x 650 nm (Fig. 6) which readily fragment. In leaf dip preparations negatively stained particles are occasionally surrounded by a loose external spiral (Fig. 7).
Particle Composition
Nucleic acid: c. 5% of particle weight (estimated spectrophotometrically).
Protein: c. 95% of particle weight (estimated spectrophotometrically); one type of polypeptide of M.Wt 32,000.
Relations with Cells and Tissues
In ultrathin sections of systemically infected soybean leaves, bundles of filamentous virus-like particles occur in the cytoplasm (M. James, R. H. Kenten & A. A. Brunt, unpublished).
Notes
Cowpea yellow mosaic virus (Chant, 1959), the bean strain of tobacco mosaic virus (Lister & Thresh, 1955) and bean southern mosaic virus (Lamptey & Hamilton, 1970) also infect cowpeas in West Africa, but cowpea mild mottle virus differs from these in particle morphology, host plant reactions and other properties. It also differs in size and/or morphology from other viruses infecting cowpeas elsewhere, including cowpea aphid-borne mosaic virus (Bock, 1973a; Bock & Conti, 1974) and peanut mottle virus (Bock, 1973b) which occur commonly in cowpea in East Africa. Cowpea mild mottle virus induces leaf symptoms in groundnuts similar to those found in plants naturally infected with similar viruses in Kenya (K. R. Bock, personal communication) and Nigeria (R. Hull, personal communication), but it is not known whether these three viruses are related.
The susceptibility of cocoa (Theobroma cacao) to infection (Brunt & Kenten, 1973) is especially interesting because infected seedlings produce leaf symptoms similar to those of an unidentified virus infecting cocoa in the Bompata area of Ashanti Akim, Ghana (Anon., 1951).
Figures
References list for DPV: Cowpea mild mottle virus (140)
- Anon., Rep. W. Afr. Cocoa Res. Inst. 1949-50: 9, 1951.
- Bock, Ann. appl. Biol. 74: 75, 1973a.
- Bock, Ann. appl. Biol, 74: 171, 1973b.
- Bock & Conti, CMI/AAB Descriptions of Plant Viruses 134, 4pp., 1974.
- Brunt & Kenten, Ann. appl. Biol. 74: 67, 1973.
- Chant, Ann. appl. Biol. 47: 565, 1959.
- Kassanis & Govier, J. gen. Virol. 10: 99, 1971a.
- Kassanis & Govier, J. gen. Virol.13: 221, 1971b.
- Lamptey & Hamilton, Phytoprotection 51: 151, 1970.
- Lister & Thresh, Nature, Lond. 175: 1047, 1955.