Details of DPV and References
DPV NO: 150 October 1975
Family: Secoviridae
Genus: Nepovirus
Species: Peach rosette mosaic virus | Acronym: PRMV
There is a more recent description of this virus: DPV 364
Peach rosette mosaic virus
H. F. Dias Research Station, Research Branch, Agriculture Canada, Vineland Station, Ontario, Canada
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by Cation (1933, 1942); Dias (1968); Ramsdell & Myers (1974).
- Selected synonyms
- Grape decline virus (Dias, 1968)
- Grapevine degeneration virus (Rev. appl. Mycol. 54, 1375)
- Grapevine degeneration virus (Rev. appl. Mycol. 54, 1375)
- An RNA-containing virus with isometric particles about 28 nm in diameter. Soil-borne, reportedly transmitted by nematodes and readily transmissible by inoculation with sap. Found naturally in peach orchards and vineyards in USA.
Main Diseases
Causes degeneration of grape (Vitis labrusca cv. Concord), and mosaic and rosetting of peach (Prunus persica).
Geographical Distribution
Only found in USA, mostly in Michigan State; a few cases reported in peaches from New York State (Hildebrand, 1941).
Host Range and Symptomatology
Found naturally in Concord grapes and peaches. The virus was recently isolated from Taraxacum officinale (dandelion) and Solanum carolinense (horse-nettle) growing in infected vineyards (D. C. Ramsdell, unpublished data). Some species of Chenopodiaceae, Cucurbitaceae, Leguminosae and Solanaceae can be infected by mechanical inoculation with sap. Chenopodium spp., particularly C. amaranticolor and C. quinoa, are very susceptible. In most herbaceous hosts the inoculated leaves are infected but remain symptomless, and symptoms on uninoculated leaves are mild and transient. Isolates differ in virulence.
- Diagnostic species
- By grafting:
- Vitis labrusca cv. Concord (grapevine). Delayed bud-break (Fig. 3), asymmetric and mottled leaves (Fig. 8), shoots with short internodes, shelling of berries and stunting of vines which may eventually die.
- Prunus persica (peach). Delayed leaf development; chlorotic mottling, wavy leaf margins and later severe distortion of leaves; extreme shortening of the internodes leading to a rosette type of growth (Fig. 4) and sometimes death of infected trees.
- By sap inoculation:
- Chenopodium amaranticolor. Faint chlorotic lesions develop in inoculated leaves within 4-7 days. Systemic symptoms may include mottling and leaf deformity, distortion and necrosis of the shoot tip (Fig. 2), and stunting of the plant within 7 to 10 days.
- C. quinoa. Faint, chlorotic local lesions in inoculated leaves within 4-7 days followed by epinasty and abscission; lesions may become necrotic in winter. Uninoculated leaves show mottle, yellow blotch and epinasty; there is severe stem twisting and frequently death of the shoot tip (Fig. 1).
- Nicotiana tabacum L. cv. Harrow Velvet. Chlorotic lesions or necrotic ringspots are induced in inoculated leaves but only by virulent strains; mild but transient systemic chlorotic ringspots occur later (Fig. 7).
- Vitis labrusca cv. Concord (grapevine). Delayed bud-break (Fig. 3), asymmetric and mottled leaves (Fig. 8), shoots with short internodes, shelling of berries and stunting of vines which may eventually die.
- Propagation species
- Chenopodium amaranticolor and C. quinoa are good hosts for
maintaining cultures. Highly infective inoculum is obtained from systemically
infected leaves of C. quinoa 8-10 days after inoculation of the plants.
- Assay species
- No reliable local lesion host has been reported, but in winter or with virulent isolates C. quinoa sometimes may be used.
Strains
Isolates from peach and grape produced reactions of complete identity in gel-diffusion tests with homologous or heterologous antisera, and shared all antigenic groups with each other in cross-absorption tests. In one instance, however, a less virulent isolate from grape contained additional antigenic determinants not present in other isolates from peach or grape as detected by cross-absorption and spur formation in gel-diffusion tests (Dias, 1972; Dias & Cation, 1975).
Transmission by Vectors
The virus is soil-borne; healthy peaches and grapes become infected when planted in infected soils (Cation, 1942, 1951). Steam treatment of infested soil from peach orchards prevented transmission (Fulton & Cation, 1959). Two nematode species, Xiphinema americanum and a Criconemoides sp., were reported to be vectors (Klos et al., 1967), but the record for Criconemoides needs confirmation. Ramsdell & Myers (1974) found large populations of X. americanum and Criconemoides xenoplax and, on occasion, low populations of Trichodorus sp. in soils from infested vineyards. Under greenhouse conditions, hand-picked X. americanum transmitted the virus from mechanically infected C. quinoa to healthy C. quinoa, but not to healthy grapes; percentage of transmission was low and erratic (H. F. Dias, unpublished data). The aphid Myzus persicae failed to transmit the virus from C. quinoa to C. quinoa irrespective of the length of the acquisition feeding period (Dias & Cation, 1975).
Transmission through Seed
Not known whether the virus is seed-borne in peach or grape. Transmitted frequently through seed of C. quinoa (Dias & Cation, 1975).
Transmission by Dodder
Not transmitted by the dodder Cuscuta campestris (Dias & Cation, 1975).
Serology
The virus is moderately immunogenic and titres of 1/256 are obtained by intramuscular injection of partially purified virus and Freund’s complete adjuvant. The virus forms a single band of precipitate in gel-diffusion serological tests.
Relationships
Peach rosette mosaic virus did not react serologically with antisera to grapevine fanleaf, grapevine chrome mosaic, tomato ringspot, tobacco ringspot, tomato black ring, tomato bushy stunt, tomato top necrosis, raspberry ringspot and prune dwarf viruses and to several strains (type, elderberry, rhubarb, dogwood) of cherry leaf roll virus.
Stability in Sap
In C. quinoa sap, the thermal inactivation point (10 min) is 58-68°C, the dilution end-point 10-3-10-5, and the virus retains infectivity for about 15-25 days at room temperaure (20°C). Sap retained some infectivity after 3 months at -15°C (Dias & Cation, 1975).
Purification
The following method gives highly infective preparations (Dias & Cation, 1975): Blend 100 g of infected C. quinoa leaves with 200 ml 0.02 M phosphate buffer (pH 7) containing 0.02 M 2-mercaptoethanol and 0.02 M Na diethyldithiocarbamate (DIECA), filter extract through cheesecloth and freeze overnight. Thaw and centrifuge at 13,000 g for 30 min and dialyse the supernatant fluid for 20 h against 5 vol 20% (w/v) ammonium sulphate. Remove precipitated material by centrifugation at 13,000 g for 30 min and concentrate the virus from the supernatant fluid by a single cycle of differential centrifugation, resuspending virus-containing pellets in 0.01 M phosphate buffer, pH 7. Pass the virus suspension through a G 200 Sephadex column using 0.017 M phosphate buffer, pH 7. Concentrate virus-containing fractions by differential centrifugation. Further purification is by rate zonal density gradient centrifugation.
Properties of Particles
Dias & Cation (1975) reported three components in purified preparations analysed by rate-zonal centrifugation in sucrose density gradients (Fig. 5). The top component (T) contained empty and disrupted virus particles; the middle (M) and bottom (B) components contained intact virus particles. When partially separated by additional gradient centrifugation M and B were both infectious and serologically related. When inoculated separately into C. quinoa both components were reproduced. The presence of M and B components in preparations was not dependent on the buffer used (pH 5.5 to pH 8), nor on the time after inoculation when the host was sampled. The virus precipitates between pH 4.0 and pH 5.0 (Dias & Cation, 1975). Sedimentation coefficient (s20,W): 52 S (T), 115 S(M), 134 S(B) (Dias & Cation, 1975).
Particle Structure
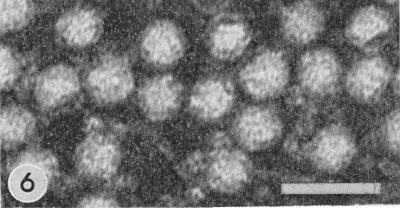
Particles are isometric and range from 24 to 31 nm, averaging 28 nm (Fig. 6). M and B components average 27 and 29 nm respectively (Dias & Cation, 1975).
Particle Composition
Unknown.
Relations with Cells and Tissues
Unknown.
Notes
Peach rosette mosaic virus can be mechanically transmitted from peach or grape to herbaceous hosts using inoculum prepared by grinding 0.2 to 0.5 g young infected leaf tissue in 5 to 10 ml of either 0.067 M phosphate buffer pH 8 (alone or containing 0.02 M DIECA), or in 2.5% nicotine solution. Transmission from grapes is best achieved by the use of nicotine solution and by subjecting the test plants to a dark period of 24 h before inoculation. Peach rosette mosaic disease is different from the peach rosette diseases described in New Zealand (Fry & Wood, 1971), Australia (Stubbs & Smith, 1971) and USA (McClintock, 1923).
Figures

Vitis labrusca cv. Concord. (Left) delayed bud-break and stunting after inoculation with peach rosette mosaic virus. (Right) healthy plant of similar age.
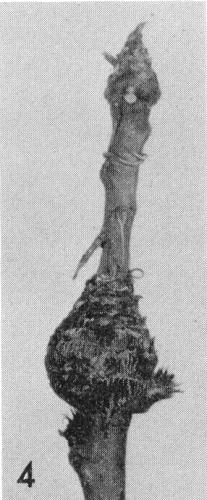
Shoot of a peach seedling with severe rosette after mechanical inoculation with peach rosette mosaic virus.
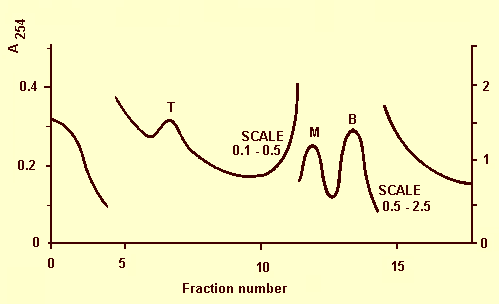
Ultraviolet absorbance scanning pattern at 254 nm of partially purified virus centrifuged for 2 h at 90,000 g in a continuous gradient of 6-60% sucrose. Sedimentation is from left to right.
References list for DPV: Peach rosette mosaic virus (150)
- Cation, Q. Bull. Mich. St. Univ. agric. Exp. Stn 16: 79, 1933.
- Cation, Tech. Bull. Mich. St. Univ. agric. Exp. Stn 180: 24 pp., 1942.
- Cation, USDA Handbook 10: 14, 1951.
- Dias, Weinberg und Keller 9: 516, 1968.
- Dias, Annls Phytopath. No. hors-série: 97, 1972.
- Dias & Cation, Can. J. Bot. 54: 1228, 1975.
- Fry & Wood, N.Z. Jl. agric. Res. 14: 515, 1971.
- Fulton & Cation, Pl. Dis. Reptr 46: 991, 1959.
- Hildebrand, Phytopathology 31: 353, 1941.
- Klos, Fronek, Knierim & Cation, Q. Bull. Mich. St. Univ. agric. Exp. Stn 49: 287, 1967.
- McClintock, J. agric. Res. 24: 307, 1923.
- Ramsdell & Myers, Phytopathology 64: 1174, 1974.
- Stubbs & Smith, Aust. J. agric. Res. 22: 771, 1971.