Details of DPV and References
DPV NO: 151 October 1975
Family: Virgaviridae
Genus: Tobamovirus
Species: Tobacco mosaic virus | Acronym: TMV
There is a more recent description of this virus: DPV 370
Tobacco mosaic virus (type strain)
M. Zaitlin Dept. of Plant Pathology, Cornell University, Ithaca, New York 14853, USA
H. W. Israel Dept. of Plant Pathology, Cornell University, Ithaca, New York 14853, USA
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Disease described by
Mayer (1886),
Iwanowski (1892) and
Allard (1914);
but this
description relates more to recent isolates of the virus for which chemical, physical
and biological measurements have been made. Exact relationships of these isolates to the
original is not known, but from the following it is presumed that all ‘type’
isolates are
very similar, if not identical. Virus obtained from W. M. Stanley
(Anderer, 1963)
was
independently sub-cultured many times in the USA and Germany, and some 20 years later
its coat protein was shown in both countries to have the same amino-acid sequence.
Selected synonyms
- Common strain, wild type, ordinary TMV, vulgare (Rev. appl. Mycol. 23:
460)
- U1 (Rev. appl. Mycol. 34: 110)
- Marmor tabaci (Rev. appl. Mycol. 28: 514)
- U1 (Rev. appl. Mycol. 34: 110)
-
An RNA-containing virus with rigid, tubular, helically symmetrical particles c. 18 x 300 nm. Wide host range, world-wide distribution. Easily transmitted by mechanical inoculation, but not by insects or other common vectors. The most investigated plant virus; important more for basic research than as an agent of economically important diseases.
Main Diseases
Elicits disease in many plant species (Smith, 1972) although exact strain not always described. Causes economically important mosaic diseases in tobacco (Nicotiana tabacum) (Gooding, 1969).
Geographical Distribution
Common in countries where tobacco is grown in the field.
Host Range and Symptomatology
Holmes (1946) obtained infection in 199 of 310 species from 30 families of angiosperms, although in 100 of these only the inoculated leaves became infected. Species differ in their capacity to support virus replication; many, including ferns (Cheo, 1972), support only very small levels (Cheo & Girard, 1971; Oxelfelt, 1974).
-
Diagnostic species
- Nicotiana tabacum
cvs. Turkish, Turkish Samsun, Samsun (Samsoun), White Burley, Burley and Xanthi. Vein-clearing appears on young, systemically-invaded leaves 3-4 days after inoculation, followed by a light green-dark green mosaic (Fig. 1), often accompanied by distortion and blistering. Inoculated leaves exhibit few symptoms other than faint chlorotic lesions when the nutrient nitrogen supply is limited. - N. glutinosa, N. tabacum cvs. Samsun NN and Xanthi-nc, Phaseolus vulgaris
cv. Pinto and Chenopodium amaranticolor form necrotic local lesions at
temperatures below c. 28°C; systemic infections appear in the Nicotiana
spp. at higher temperatures.
- N. sylvestris, and N. tabacum cv. Java develop systemic infections; however, strains or mutants may produce necrotic local lesions without systemic infection.
-
Propagation species
- N. tabacum
cvs. Turkish, Turkish Samsun, Samsun or Xanthi.Assay species
- Local lesion assays are most frequently performed with N. tabacum cvs. Xanthi-nc or Samsun NN, N. glutinosa, Phaseolus vulgaris cv. Pinto or Chenopodium amaranticolor.
Strains
Hennig & Wittmann (1972) list many well-documented tobamoviruses; some are the subject of other Descriptions in this series. Tobacco plants infected with type strain may ‘give rise’ to variants which can be isolated from yellow or necrotic spots in systemically-invaded tissue. A ‘masked’ strain inducing no symptoms in Samsun tobacco has been selected by growing type strain-infected plants at 35°C (Holmes, 1934). Many chemically induced mutants have been isolated (Hennig & Wittmann, 1972).
Transmission by Vectors
Considered not normally transmissible by arthropods but is readily spread between plants by contact and by man during cultural operations. Scattered reports of insects as vectors (Lojek & Orlob, 1969; Harris & Bradley, 1973) relate to their acting inadvertently as agents of mechanical inoculation. Soil-borne virus particles or fragments of infected tissue can serve as sources of infection via roots.
Transmission through Seed
Type strain not transmissible by seed or pollen.
Transmission by Dodder
Transmitted, chiefly among tobaccos, by Cuscuta campestris, C. japonica and C. subinclusa (Hosford, 1967) but the specific virus strains have not always been identified.
Serology
Good immunogen; high titred antisera can be obtained with either whole virus or coat protein subunits. Easily detected by interfacial ring precipitation tests, quantitative precipitin tests, radioimmunoassay, inhibition of infectivity by serum, etc., and by a variety of gel-diffusion procedures (Ball, 1974). Gel-diffusion reactions with intact virus develop only after several days because the antigen is large. Topic reviewed by Rappaport (1965) and van Regenmortel (1966).
Relationships
Strains and mutants exhibit differing amounts of serological cross reactivity with type strain, depending on the nature and position of amino acid replacements in the coat protein (von Sengbusch, 1965; van Regenmortel, 1967). Some symptom variants, including the masked strain, seem serologically identical with the type strain. Relationships to tomato mosaic, U2 and other tobamovirus isolates are being dealt with in a separate Description.
Stability in Sap
Very stable; preparations retain infectivity for decades (Silber & Burk, 1965). Also very heat-stable; some infectivity is retained after 10 min exposures at over 90°C. Dilutions of 10-6 of expressed tobacco sap can be infectious.
Purification
Because of its high titre and stability, TMV can be purified by many procedures such as ultracentrifugation, or salt, isoelectric or solvent precipitations. The polyethylene glycol procedure of Gooding & Hebert (1967) also yields satisfactory preparations; modifications include the use of buffered 5% Triton X-100 to eliminate chloroplast fragments (Nozu & Yamura, 1971) and incubation of virus with chelating agents to remove coloured materials (Ginoza et al., 1954). Resuspension of sedimented virus in dilute ethylenediamine-tetraacetate (EDTA) reduces virus aggregation (Boedtker & Simmons, 1958). Particles can be sorted according to length using columns of agar or agarose beads (Steere, 1963). Yields may reach 10 mg/g fresh weight, but 1-3 are more common.
Properties of Particles
Sedimentation coefficient (s20, w) at infinite dilution is c. 194 S (Harrington & Schachman, 1956).
M. Wt is c. 39.4 x 106 daltons (Caspar, 1963).
Diffusion coefficient (D20,w) is c. 4.4 x 10-8 cm2/sec (Schramm & Bergold, 1947).
Isoelectric point is c. pH 3.5 (Fraenkel-Conrat & Narita, 1958).
Partial specific volume is c. 0.73 cm3/gm (Lauffer, 1944).
Electrophoretic mobility at ionic strength (G) 0.075 and pH 6.5-7.9 is c. -0.83 (µm/sec)/(V/cm) (Kramer & Wittmann, 1958).
Extinction coefficient (A(0.1%,1 cm), at 260 nm, uncorrected for light scattering, ranges between 2.7 and 3.5 (Brakke, 1967); a value of 3.0 is commonly used.
A260/A280 is c. 1.19 (Paul, 1958).
Buoyant density in CsCl is c. 1.325 (Siegel & Hudson, 1959).
Particle Structure
Straight, rigid tubules; length c. 300 nm, max. radius c. 9 nm; composed of c. 2130 identical protein subunits closely packed in a helix (pitch c. 2.3 nm, 16 1/3 subunits/turn) around a cylindrical canal of radius c. 2 nm. One continuous single strand of RNA, of c. 6390 nucleotides, follows the same helix (49 nucleotides/turn or 3/subunit) at a radius of c. 4 nm, and is associated with the protein subunits near their inner surfaces (Caspar, 1963) (Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10). Particles can be dissociated into constituent nucleic acid and protein and reconstituted into stable infective viral particles (Fraenkel-Conrat, 1970).
Particle Composition
Nucleic acid. RNA, single-stranded, M. Wt 2.05 x 106, c. 5% of particle weight. G:A:C:U = 25.3:29.8:18.5:26.3 (Caspar, 1963). No subsidiary RNA components in virus particle, but pieces of viral RNA occur in tissues (Siegel et al., 1973). Preparation of infective RNA described by Mandeles & Bruening (1968); s20,w is 32.5 S in 0.05 M KCl, 2 mM EDTA, 5 µg/ml sodium dodecyl sulphate.
Protein: Can be isolated according to Fraenkel-Conrat (1957). About 95% of particle; subunits, of M. Wt c. 1.75 x 104, each consist of a chain of 158 amino acids with the following sequence (Wittmann-Liebold & Wittmann, 1967):
10
AcSer -Tyr-Ser-Ile-Thr-Thr-Pro-Ser-Gln-
Phe-Val-Phe-Leu-Ser-Ser-Ala-Trp-Ala-Asp-
20 30
Pro-Ile-Glu-Leu-Ile-Asn-Leu-Cys-Thr-Asn-
Ala-Leu-Gly-Asn-Gln-Phe-Gln-Thr-Gln-Gln-
40 50
Ala-Arg-Thr-Val-Val-Gln-Arg-Gln-Phe-Ser-
Gln-Val-Trp-Lys-Pro-Ser-Pro-Gln-Val-Thr-
60 70
Val-Arg-Phe-Pro-Asp-Ser-Asp-Phe-Lys-Val-
Tyr-Arg-Tyr-Asn-Ala-Val-Leu-Asp-Pro-Leu-
80 90
Val-Thr-Ala-Leu-Leu-Gly-Ala-Phe-Asp-Thr-
Arg-Asn-Arg-Ile-Ile-Glu-Val-Glu-Asn-Gln-
100 110
Ala-Asn-Pro-Thr-Thr-Ala-Glu-Thr-Leu-Asp-
Ala-Thr-Arg-Arg-Val-Asp-Asp-Ala-Thr-Val-
120 130
Ala-Ile-Arg-Ser-Ala-Ile-Asn-Asn-Leu-Ile-
Val-Glu-Leu-Ile-Arg-Gly-Thr-Gly-Ser-Tyr-
140 150
Asn-Arg-Ser-Ser-Phe-Glu-Ser-Ser-Ser-Gly-
Leu-Val-Trp-Thr-Ser-Gly-Pro-Ala-Thr-COOH
Note: In the Japanese common strain (OM) the threonine at position 153 is replaced by asparagine and the isoleucine at position 129 is replaced by valine (Nozu et al., 1970).
Particles have no known enzymatic activity, but RNase is a troublesome contaminant (Whitfeld & Williams, 1963).
Lipid: None.
Other components: Traces of metal (Loring et al., 1962) and a polyamine (Johnson & Markham, 1962), the nature of which is challenged (Beer & Kosuge, 1970), are reported as possible constituents of the virus particle.
Relations with Cells and Tissues
Many kinds of cell are infected. Particles are mainly in the cytoplasm and may associate with all major organelles including cell walls (Esau, 1968); those in chloroplasts, however, may not be true virus particles (Shalla et al., 1975).
Virus-induced RNA replicase of M. Wt c. 1.6 x 105 (Ralph & Wojcik, 1969; Zaitlin et al., 1973) and double-stranded RNA (Nilsson-Tillgren et al., 1974) are associated with cytologically unspecified cell membranes. Virus coat protein can be associated with chloroplasts (Shalla et al., 1975), ground cytoplasm and nuclei (Langenberg & Schlegel, 1969; Shalla & Amici, 1967). Synthesis of several proteins is stimulated in infected cells (Zaitlin & Hariharasubramanian, 1972). Nevertheless, evidence for sites of synthesis or assembly of the virus is equivocal.
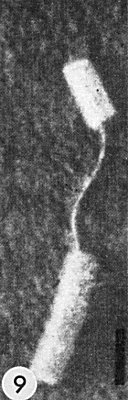
Virus-related inclusions, visible by light and electron microscopy, range from hexagonal crystal line plates of virus particles (Fig. 3) to lateral aggregates of closely packed particles (Fig. 4) to linear aggregates of needles, spindles and fibres, and very large unbounded, amorphous and vacuolated X-bodies - of unknown function - composed variously of virus particles, proteinaceous granules and tubules together with host organelles (Esau, 1968) (Fig. 2).
Notes
Type strain used in many laboratories throughout the world apparently has a common origin. Personal recollections of C. A. Knight, W. C. Price and F. O. Holmes suggest that the original isolate used by W. M. Stanley came from J. Johnson of the Univ. of Wisconsin via L. O. Kunkel. The U1 strain (Siegel & Wildman, 1954) and the German strain ‘vulgare’ (Wittmann-Liebold & Wittmann, 1967) also came from Johnson. TMV was the first virus to be purified (Stanley, 1935), shown to contain RNA (Bawden & Pirie, 1937), reassembled from its constituents (Fraenkel-Conrat & Williams, 1955), and used for production of chemically-induced mutants as a confirmation of the genetic code (Gierer & Mundry, 1958).
Figures
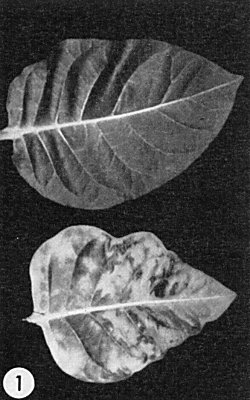
Leaves from healthy (upper) and systemically infected (lower) plants of Nicotiana tabacum cv. Turkish. (Courtesy A. F. Ross.)

Hexagonal virus crystal (left) and cell nucleus (right) in leaf-hair cell of N. tabacum. Bar represents 6 µm.

Lateral aggregates of virus in layered ranks in parenchyma cell of N. tabacum (courtesy K. Esau, by permission of Univ. of Wisconsin Press). Bar represents 670 nm.
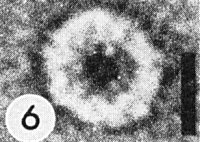
End view of virus fragment, negatively stained with vanadatomolybdate, showing 3 concentric regions. Bar represents 10 nm.
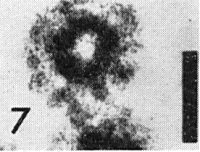
Transverse section of virus particle in parenchyma cell of N. tabacum (courtesy K. Esau, by permission of Univ. Wisconsin Press). Bar represents 11 nm.
References list for DPV: Tobacco mosaic virus (type strain) (151)
- Allard, Dep. Bull. U.S. Dep. Agric. 40, 33 pp., 1914.
- Anderer, Adv. Protein Chem. 18: 1, 1963.
- Ball, Serological Tests for the Identification of Plant Viruses, American Phytopathological Society, St. Paul, 1974.
- Bawden & Pirie, Proc. Roy. Soc. Lond. B 123: 274, 1937.
- Beer & Kosuge, Virology 40: 930, 1970.
- Boedtker & Simmons, J. Am. Chem. Soc. 80: 2550, 1958.
- Brakke, in Methods in Virology, Vol. 2, Eds. K. Maramorosch & H.Koprowski, Academic Press: New York, p. 104, 1967.
- Caspar, Adv. Protein Chem. 18: 37, 1963.
- Cheo, Pl. Dis. Reptr 56: 1010, 1972.
- Cheo & Girard, Phytopathology 61: 1010, 1971.
- Esau, Viruses in Plant Hosts, Univ. of Wisconsin Press, Madison, 1968.
- Fraenkel-Conrat, Virology 4: 1, 1957.
- Fraenkel-Conrat, A. Rev. Microbiol. 24: 463, 1970.
- Fraenkel-Conrat & Narita, in Symposium on Protein Structure, Ed. A.Neuberger, Methuen, London, p. 249, 1958.
- Fraenkel-Conrat & Williams, Proc. Natl. Acad. Sci. U.S. 41: 690, 1955.
- Gierer & Mundry, Nature, Lond. 182: 1457, 1958.
- Ginoza, Atkinson & Wildman, Science, N.Y. 119: 269, 1954.
- Gooding, Tech. Bull. N. Carol. agric. Exp. Stn 195, 24 pp., 1969.
- Gooding & Hebert, Phytopathology 57: 1285, 1967.
- Harrington & Schachman, Arch. Biochem. Biophys. 65: 278, 1956.
- Harris & Bradley, Virology 52: 295, 1973.
- Hennig & Wittmann, in Principles and Techniques in Plant Virology, Eds. C. Kado & H. Agrawal, New York, Van Nostrand Reinhold, p. 546, 1972.
- Holmes, Phytopathology 24: 845, 1934.
- Holmes, Phytopathology 36: 643, 1946.
- Hosford, Bot. Rev. 33: 387, 1967.
- Iwanowski, Izv. imp. Akad. Nauk 35: 67, 1892, Translated into English as Phytopathological Classics No. 7, American Phytopathological Society, Minneapolis, 1942.
- Johnson & Markham, Virology 17: 276, 1962.
- Kramer & Wittmann, Z. Naturforsch. B 13: 30, 1958.
- Langenberg & Schlegel, Virology 37: 86, 1969.
- Lauffer, J. Am. Chem. Soc. 66: 1188, 1944.
- Lojek & Orlob, Science, N.Y. 164: 1407, 1969.
- Loring, Fujimoto & Tu, Virology 16: 30, 1962.
- Mandeles & Bruening, Biochem. Prep. 12: 111, 1968.
- Mayer, Landwn. VersStnen 32: 451, 1886, Translated into English as Phytopathological Classics No. 7, American Phytopathological Society, Minneapolis, 1942.
- Nilsson-Tillgren, Kielland-Brandt & Bekke, Molec. gen. Genetics 128: 157, 1974.
- Nozu & Yamura, Virology 43: 514, 1971.
- Nozu, Ohno & Okada, J. Biochem. 68: 39, 1970.
- Oxelfelt, Phytopath. Z. 79: 281, 1974.
- Paul, Arch. Mikrobiol. 30: 304, 1958.
- Ralph & Wojcik, Virology 37: 276, 1969.
- Rappaport, Adv. Virus Res. 11: 223, 1965.
- Schramm & Bergold, Z. Naturforsch. B 2: 108, 1947.
- Shalla & Amici, Virology 31: 78, 1967.
- Shalla, Peterson & Giunchedi, Virology 66: 94, 1975.
- Siegel & Hudson, Biochim. Biophys. Acta 34: 254, 1959.
- Siegel & Wildman, Phytopathology 44: 277, 1954.
- Siegel, Zaitlin & Duda, Virology 53: 75, 1973.
- Silber & Burk, Nature, Lond. 206: 740, 1965.
- Smith, A Textbook of Plant Virus Diseases, 3rd Ed., Longman, London, 1972.
- Stanley, Science, N.Y. 81: 644, 1935.
- Steere, Science, N.Y. 140: 1089, 1963.
- van Regenmortel, Adv. Virus Res. 12: 207, 1966.
- van Regenmortel, Virology 31: 467, 1967.
- von Sengbusch, Z. Vererbungsl. 96: 364, 1965.
- Whitfeld & Williams, Virology 21: 156, 1963.
- Wittmann-Liebold & Wittmann, Molec. gen. Genetics 100: 358, 1967.
- Zaitlin & Hariharasubramanian, Virology 47: 296, 1972.
- Zaitlin, Duda & Petti, Virology 53: 300, 1973.