Details of DPV and References
DPV NO: 153 October 1975
Family: Virgaviridae
Genus: Tobamovirus
Species: Sunn-hemp mosaic virus | Acronym: SHMV
Sunn-hemp mosaic virus
B. Kassanis Rothamsted Experimental Station, Harpenden, Hertfordshire, England
A. Varma Division of Mycology and Plant Pathology, Indian Agr. Research Institute, New Delhi-110012, India
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by
Capoor & Varma (1948),
Capoor (1950;
1962),
Lister & Thresh (1955).
Selected synonyms
- Dolichos enation mosaic virus (Rev. appl. Mycol. 27: 309)
- Southern sunn-hemp mosaic virus (Rev. appl. Mycol. 29: 413)
- Crotalaria mucronata mosaic virus (Rev. appl. Mycol. 29: 569)
- Cowpea mosaic virus of Lister & Thresh (Rev. appl. Mycol. 34: 624)
- Southern sunn-hemp mosaic virus (Rev. appl. Mycol. 29: 413)
-
An RNA-containing virus with rod-shaped particles 300 nm long, readily sap-transmissible, having a wide host range especially among legumes. Serologically related to tobacco mosaic virus. No known vector.
Main Diseases
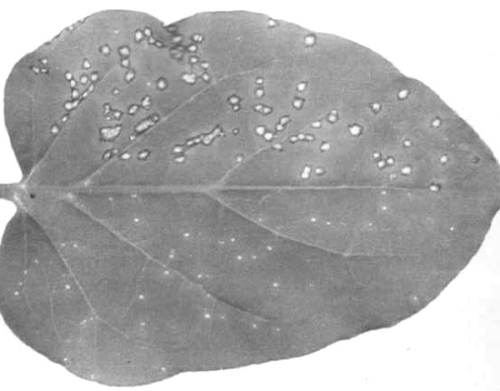
In sunn-hemp (Crotalaria juncea) causes severe mosaic, puckering, blistering and malformation (Fig. 2) with enations on the undersides of the leaves. Plants are stunted (Capoor, 1950, 1962). Dolichos lablab (Capoor & Varma, 1948), cowpea (Vigna unguiculata) and Bengal bean (Mucuna aterrima) (Lister & Thresh, 1955) are affected similarly.
Geographical Distribution
India (Capoor, 1950), Nigeria (Lister & Thresh, 1955) and USA (Toler, 1964).
Host Range and Symptomatology
Natural hosts are legumes. More than 50 species in 6 dicotyledonous families have been infected experimentally (Capoor, 1962).
-
Diagnostic species
- Phaseolus vulgaris
(French bean cv. The Prince). White or yellow local lesions in inoculated primary leaves, vein-clearing in young systemically infected trifoliate leaves, followed by severe mosaic, blistering, leaf deformities and enations (Fig. 1). - Nicotiana glutinosa. Necrotic local lesions less than 1 mm in diameter
appearing 4 days after inoculation
(Fig. 4)
(Bawden, 1958).
- Cyamopsis tetragonoloba (guar). Distinct dark brown lesions (Fig. 3); the number produced is much increased by keeping the plants in the dark prior to inoculation (Capoor, 1962).
-
Propagation species
- Phaseolus vulgaris
primary leaves collected 10 days after inoculation.Assay species
- Nicotiana glutinosa
or, better, N. tabacum cv. Xanthi-nc.
Transmission by Vectors
No vector has been found (Capoor & Varma, 1948). Claims that cowpea (chavali) mosaic virus is transmitted by various aphids in the stylet-borne manner (Capoor et al., 1947; Nariani & Kandaswamy, 1961; Haque & Chenulu, 1967) and by the beetle Ootheca mutabilis (Chant, 1959), are thought to refer to viruses other than sunn-hemp mosaic virus.
Transmission through Seed
Little or no transmission of typical isolates through seeds of sunn-hemp (Capoor, 1962; Nagaich & Vashisth, 1963; Capoor et al., 1947), but the cowpea chlorotic spot isolate is transmitted to 4-20% of seed of Vigna sinensis cv. Pusa Dophasli (S. R. Sharma & A. Varma, unpublished results).
Transmission by Dodder
No information.
Serology
The virus is strongly immunogenic. It reacts with antiserum to give flocculent precipitates in tube-precipitin tests, and two lines of precipitate in gel-diffusion tests (Kassanis & McCarthy, 1967).
Relationships
Sunn-hemp, cowpea and dolichos isolates are serologically very similar if not identical (Badami, 1963; B. Kassanis, unpublished results) and have the same amino acid composition (Rees & Short, 1965, 1972; J. Carpenter, unpublished results). There is also a close relationship with cowpea chlorotic spot virus (S. R. Sharma & A. Varma, unpublished results). Serological tests between different isolates have also been reported by Anand & Sahambi (1965). Sunn-hemp mosaic virus has few antigenic determinants in common with the type strain of tobacco mosaic virus or with other well studied tobamoviruses. In cross absorption tests with type strain tobacco mosaic virus the antiserum titre for the homologous virus is not affected (Bawden & Kassanis, 1968).
Stability in Sap
In sap from sunn-hemp plants assayed on N. glutinosa the dilution end-point was 10-6-10-7, the thermal inactivation point (10 min) was 90°-95°C and infectivity survived for over 8 years at 20-32°C (Capoor, 1962; Capoor & Varma, 1948).
Purification
Extract leaves in a blender with a minimal volume of 0.2 M phosphate buffer pH 5.2 to prevent particles disrupting. Squeeze sap through muslin and concentrate by two or three cycles of differential centrifugation (10 min at 10,000 g; 1.5 h at 100,000 g), resuspending virus pellets in water. Dialyse the final preparation against distilled water (Kassanis & McCarthy, 1967).
Properties of Particles
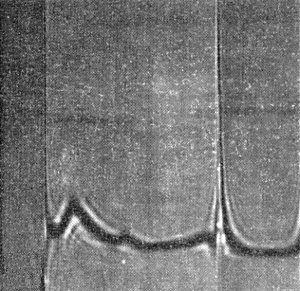
In 0.06 M phosphate buffer, pH 8, the virus sediments as 3 components of different particle length with sedimentation coefficients (s20, w) of 20-50 S, 70-80 S and 187 S (Fig. 6). Only the particles of 187 S are infective (Kassanis & McCarthy, 1967).
Electrophoretic mobility: Electrophoresis of virus at 2 mg/ml in 0.067 M phosphate buffer, pH 7, gives two components; the major one has a mobility of -4.7 x 10-5 cm2 sec-1 volt-1 and the minor one -9.4; the second might be caused by contamination with the type strain of tobacco mosaic virus (Fig. 7; Bawden, 1958).
Absorbance at 260 nm (1 mg/ml, 1 cm light path): 3.2.
A260/A280: 1.2.
Particle Structure
Rod-shaped particles 300 nm long and 17 nm wide, but preparations always contain many shorter particles (Fig. 5). A larger proportion of 300 nm particles is produced when plants are grown at 32°C (Kassanis & McCarthy, 1967).
Particle Composition
Nucleic acid: Not investigated but probably resembles the RNA of the type strain of tobacco mosaic virus.
Protein: The molecular weight of the coat protein is 18,062. The amino acid sequence (161 residues) is:
10
AcAla -Tyr-Ser-Ile-Pro-Thr-Pro-Ser-Gln-
Leu-Val-Tyr-Phe-Thr-Glu-Asn-Tyr-Ala-Asp-
20 30
Tyr-Ile-Pro-Phe-Val-Asn-Arg-Leu-Ile-Asn-
Ala-Arg-Ser-Asn-Ser-Phe-Gln-Thr-Gln-Ser-
40 50
Gly-Arg-Asp-Glu-Leu-Arg-Glu-Ile-Leu-Ile-
Lys-Ser-Gln-Val-Ser-Val-Val-Ser-Pro-Ile-
60 70
Ser-Arg-Phe-Pro-Ala-Glu-Pro-Ala-Tyr-Tyr-
Ile-Tyr-Leu-Arg-Asp-Pro-Ser-Ile-Ser-Thr-
80 90
Val-Tyr-Thr-Ala-Leu-Leu-Glu-Ser-Thr-Asp-
Thr-Arg-Asn-Arg-Val-Ile-Glu-Val-Glu-Asn-
100 110
Ser-Thr-Asp-Val-Thr-Thr-Ala-Glu-Gln-Leu-
Asn-Ala-Val-Arg-Arg-Thr-Asp-Asp-Ala-Ser-
120 130
Thr-Ala-Ile-His-Asn-Asn-Leu-Glu-Gln-Leu-
Leu-Ser-Leu-Leu-Thr-Asn-Gly-Thr-Gly-Val-
140 150
Phe-Asn-Arg-Thr-Ser-Phe-Glu-Ser-Ala-Ser-
Gly-Leu-Trp-Leu-Val-Thr-Thr-Pro-Thr-Arg-
160
Thr-Ala.
There are 96 amino acid changes from the type strain of tobacco mosaic virus (Rees & Short, 1975; Rees et al., 1974).
Relations with Cells and Tissues
Amorphous and crystalline inclusions have been found in infected Dolichos lablab cells (Sulochana & Solomon, 1970).
Notes
Other viruses have been reported causing similar symptoms in sunn-hemp (Raychaudhuri, 1947; Das Gupta et al., 1951; Das & Raychaudhuri, 1963; van Velsen & Crowley, 1962). Bawden(1956, 1958) claimed that sunn-hemp mosaic virus changed into the type strain of tobacco mosaic virus when inoculated to tobacco plants and reverted back to the original virus when inoculated to French bean. B. Kassanis (unpublished results) could not confirm this reversible change using Bawden’s inocula in 300 inoculations to tobacco plants and 500 inoculations to French bean.
Acknowledgements
Figs 2 and 3 Courtesy of I.A.R.I., New Delhi.
Figures
References list for DPV: Sunn-hemp mosaic virus (153)
- Anand & Sahambi, Indian Phytopath. 18: 204, 1965.
- Badami, Bull. natn. Inst. Sci. India 24: 166, 1963.
- Bawden, Nature, Lond. 177: 302, 1956.
- Bawden, J. gen. Microbiol. 18: 751, 1958.
- Bawden & Kassanis, Virology 34: 174, 1968.
- Capoor, Curr. Sci. 19: 22, 1950.
- Capoor, Phytopathology 52: 393, 1962.
- Capoor & Varma, Curr. Sci. 17: 57, 1948.
- Capoor & Varma, Indian J. agric. Sci. 26: 95, 1956.
- Capoor, Varma & Uppal, Curr. Sci. 16: 151, 1947.
- Chant, Ann. appl. Biol. 47: 565, 1959.
- Das & Raychaudhuri, Indian Phytopath. 16: 214, 1963.
- Das Gupta, De & Raychaudhuri, Nature, Lond. 168: 114, 1951.
- Haque & Chenulu, Phytopath. Z. 59: 277, 1967.
- Kassanis & McCarthy, J. gen. Virol. 1: 425, 1967.
- Lister & Thresh, Nature, Lond. 175: 1047, 1955.
- Nagaich & Vashisth, Indian J. Microbiol. 3: 113, 1963.
- Nariani & Kandaswamy, Indian Phytopath. 14: 77, 1961.
- Raychaudhuri, Curr. Sci. 16: 26, 1947.
- Raychaudhuri, Nariani & Das, Indian Phytopath. 15: 79, 1962.
- Rees & Short, Virology 26: 596, 1965.
- Rees & Short, Virology 50: 772, 1972.
- Rees & Short, Biochim. biophys. Acta 393: 15, 1975.
- Rees, Short, Self & Eagles, Biomedical Mass Spectrometry 1: 237, 1974.
- Sulochana & Solomon, Proc. Indian Acad. Sci. Sect. B. 71: 56, 1970.
- Toler, Phytopathology 54: 910, 1964.
- van Velsen & Crowley, Aust. J. agric. Res. 13: 220, 1962.