Details of DPV and References
DPV NO: 159 September 1976
Family: Secoviridae
Genus: Cheravirus
Species: Cherry rasp leaf virus | Acronym: CRLV
Cherry rasp leaf virus
R. Stace-Smith Agriculture Canada Research Station, Vancouver, B.C., Canada
A. J. Hansen Agriculture Canada Research Station, Summerland, B.C., Canada
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by Bodine & Newton (1942).
- Synonym
- Flat apple virus (Rev. appl. Mycol. 46, 2772).
- An RNA-containing virus with isometric particles about 30 nm in diameter occurring in western North America. It is readily transmitted by inoculation of sap and has a wide host range, including both herbaceous and woody plants. The virus is transmitted by the nematode Xiphinema americanum. It is seed-transmitted in some herbaceous hosts.
Main Diseases
The virus causes two diseases, cherry rasp leaf and flat apple. It infects cherry (Prunus avium and P. mahaleb) and peach (P. persica) but does not usually become fully systemic. Causes rasp-like enations on the underside of cherry leaves (Fig. 1), small enations, stunted growth and shortened internodes in peach, and a general decline in cherry and peach. Affected cherry branches become highly frost-sensitive (Bodine, Blodgett & Lott, 1951; Hansen et al., 1974; Wagnon et al., 1968). Also sometimes causes enations on apple leaves (Lott & Keane, 1960) and flattened apple fruits on some cultivars (Parish & Cheney, 1974). The virus does not induce symptoms in naturally-infected balsamroot (Balsamorhiza sagittata), dandelion (Taraxacum officinale) and plantain (Plantago major).
Geographical Distribution
North America, in the foothills and west of the Rocky Mountains, from Colorado, Utah and California to southern British Columbia.
Host Range and Symptomatology
Natural infection has been found in native balsam-root, in dandelion and plantain under orchard trees, in scattered trees or small groups of cherry and peach, and in several apple orchards in Washington State, USA. The virus infected 22 out of 24 commonly-used herbaceous plants. Symptoms were generally mild and often absent. Isolates differ in the severity of symptoms they induce, but not in host range.
- Diagnostic species
- Cucumis sativus (cucumber). Faint chlorotic primary lesions in
cotyledons; fine systemic mottle (Fig. 2).
- Cyamopsis tetragonoloba (guar). Brown necrotic lesions after 4 (summer) to 14 (winter) days in inoculated cotyledons; no systemic infection.
- Chenopodium quinoa. A fine mottle and vein clearing on the third or fourth leaf above the inoculated leaf (Fig. 3). Wilting of axillary shoots (under winter greenhouse conditions only).
- C. amaranticolor. Systemic mottle about 7 days after inoculation (Fig. 4).
- Vigna sinensis (cowpea), Physalis floridana and Sesbania exalta usually display lesions in inoculated leaves but are unreliable under unfavourable greenhouse conditions (Hansen et al., 1974).
- Cyamopsis tetragonoloba (guar). Brown necrotic lesions after 4 (summer) to 14 (winter) days in inoculated cotyledons; no systemic infection.
- Assay species
- Cucumis sativus is a reliable local lesion host. Cyamopsis tetragonoloba may also be useful.
- Propagation species
- C. quinoa, Cucumis sativus (cucumber).
Strains
Minor differences between strains were observed in symptom severity on herbaceous hosts and in lesion type on guar (Hansen et al., 1974).
Transmission by Vectors
Transmitted by the nematode Xiphinema americanum from Chenopodium amaranticolor and C. quinoa to Chenopodium bait plants (Nyland et al., 1969). Transmitted to cucumber bait plants and mazzard cherry seedlings which received viruliferous X. americanum from affected orchard and nursery trees (Hansen et al., 1974).
Transmission through Seed
10-20% of seeds from infected Chenopodium quinoa and Taraxacum officinale plants gave rise to infected plants. Seeds from infected parts of cherry trees did not germinate. Virus can be detected in pollen from infected cherry trees by mechanical transmission tests (Wagnon et al., 1968).
Transmission by Dodder
Not tested.
Serology
The virus is moderately immunogenic-antisera with titres of 1/640 are readily obtained. Antisera prepared by intravenous and intramuscular injections give a single band of precipitate in gel-diffusion tests. Agar gel tests on microscope slides are recommended. Virus can be detected serologically in crude sap extracted from inoculated cucumber cotyledons.
Relationships
The isolates that were studied were serologically identical. In many of its properties it resembles nepoviruses (tobacco ringspot, tomato ringspot, cherry leaf roll, peach rosette mosaic, raspberry ringspot, tomato black ring and arabis mosaic) but it is unrelated to them serologically (Hansen et al., 1974), and its protein components are anomalous in size.
Stability in Sap
In Cucumis sativus sap, the virus loses infectivity after 10 min at about 58°C or 7 days at 4°C. Dilution end-point is c. 10-4 (Stace-Smith & Hansen, 1976).
Purification
The virus is relatively unstable and is degraded by purification procedures commonly used with nepoviruses. Virus purifications have been attempted from infected cucumber, C. quinoa and N. clevelandii and, of these, cucumber is the preferred source. The following method has given yields of 10 mg/kg of infected tissue: homogenize tissue in cold 0.5 M borate, 0.05 M EDTA buffer, containing 0.02 M mercaptoethanol and adjusted to pH 6.5, 2 ml/g of tissue. Squeeze through muslin and adjust to pH 6.5 with citric acid. Centrifuge at low speed, add granular ammonium sulphate, 15 g/100 ml, to supernatant fluid, and stir overnight. Centrifuge at low speed, retaining supernatant fluid, and follow with high speed centrifugation, resuspending pellet in 0.05 M borate buffer, pH 6.5. The resuspended material may be further purified by density gradient centrifugation (Stace-Smith & Hansen, 1976).
Properties of Particles
Purified preparations contain three classes of particles, empty protein shells without RNA (T) sedimenting at 56 S and two nucleoproteins (M and B) sedimenting at 96 and 128 S (Stace-Smith & Hansen, 1976).
Particle Structure
Isometric, about 30 nm in diameter, usually hexagonal in outline (Fig. 5).
Particle Composition
- Nucleic acid: RNA, single-stranded. Combined M and B components
contain two RNA species with M. Wt c. 2.0 x 106and 1.5 x 106.
- Protein: Particles contain two kinds of polypeptide molecules of M. Wt 24,000 and 22,500 (Stace-Smith & Hansen, 1976).
Relations with Cells and Tissues
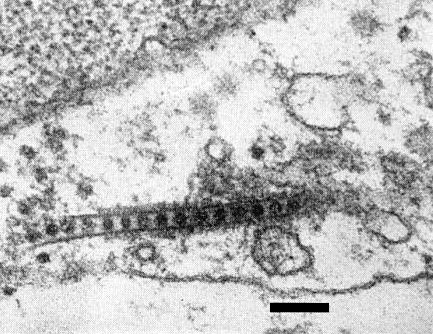
In systemically infected cucumber and C. quinoa, rows of virus particles enclosed within a tubular structure are found within the cytoplasm of parenchyma cells (Fig. 6). Particles are also frequently found within the plasmodesmata (Stace-Smith & Hansen, 1976).
Notes
The virus occurs in a wide geographical area but primary outbreaks are usually limited to one or very few trees. Secondary spread is generally slow, due to the slow lateral movement of the nematode vector. However, in older cherry-growing areas, rasp leaf infection can be as high as 38% (Luepschen et al., 1974). Cherry or apple trees planted on the site of previously-infected trees often become infected (Wagnon et al., 1968; Parish & Cheney, 1974). Cherry rasp leaf is unrelated to Eola rasp leaf and other cherry diseases characterized by development of leaf enations.
Figures
References list for DPV: Cherry rasp leaf virus (159)
- Bodine & Newton, Phytopathology 32: 333, 1942.
- Bodine, Blodgett & Lott, Handb. U.S. Dept. Agric. 10: 71-80, 1951.
- Hansen, Nyland, McElroy & Stace-Smith, Phytopathology 64: 721, 1974.
- Lott & Keane, Pl. Dis. Reptr 44: 634, 1960.
- Luepschen, Harder, Rohrback & Sisson, Pl. Dis. Reptr 58: 26, 1974.
- Nyland, Lownsbery, Lowe & Mitchell, Phytopathology 59: 1111, 1969.
- Parish & Cheney, Proc. Am. phytopath. Soc. 1: 52, 1974.
- Stace-Smith & Hansen, Acta Hort. 67: 193, 1976.
- Wagnon, Traylor, Williams & Weiner, Pl. Dis. Reptr 52: 618, 1968.