Details of DPV and References
DPV NO: 160 September 1976
Family: Secoviridae
Genus: Nepovirus
Species: Myrobalan latent ringspot virus | Acronym: MLRSV
Myrobalan latent ringspot virus
J. Dunez Station de Pathologie Végétale, Centre de Recherches de Bordeaux, 33140 Pont-de-la-Maye, France
R. Delbos Station de Pathologie Végétale, Centre de Recherches de Bordeaux, 33140 Pont-de-la-Maye, France
G. Dupont Laboratoire de Biologie Cellulaire et Moléculaire, Centre de Recherches de Bordeaux, 33140 Pont-de-la-Maye, France
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by Dunez et al. (1971).
- An isometric RNA-containing virus 28 nm in diameter with two sedimenting nucleoprotein components. It is transmissible by inoculation of sap and is serologically related to the nepovirus, tomato black ring. It occurs naturally in some rootstocks of Prunus cerasifera Myrobalan B and in Prunus cultivars grafted on this rootstock. Found in France.
Main Diseases
Latent in myrobalan (Prunus cerasifera). It causes short internodes and rosetting in peach (Prunus persica) and enations on the leaves of sweet cherry (P. avium) cv. Bing (Fig. 1).
Geographical Distribution
Found only in south west France.
Host Range and Symptomatology
Many species of Prunus develop no symptoms when infected. However, infected plants of some cultivars of P. avium, especially Bing, develop enations on the underside of the leaves (Fig. 1) and peach develops severe rosetting. Additional hosts in the Solanaceae, Chenopodiaceae, Compositae, Cucurbitaceae and Papilionaceae can be infected by inoculation of sap. Inoculum from Prunus should be made by extracting the leaves in 2% nicotine.
- Diagnostic species
- Chenopodium amaranticolor, C. quinoa. Necrotic local lesions 3 days
after inoculation. Systemic necrosis (Fig. 2) is followed by partial recovery.
- Nicotiana tabacum cv. Xanthi-nc. A few necrotic spots in the inoculated leaves. Systemically infected leaves develop typical ringspot symptoms (Fig. 3).
- Nicotiana tabacum cv. Xanthi-nc. A few necrotic spots in the inoculated leaves. Systemically infected leaves develop typical ringspot symptoms (Fig. 3).
- Propagation species
- C. quinoa is used as a source of virus for purification. Cultures
are maintained in this plant, in Nicotiana clevelandii or in Pisum
sativum.
- Assay species
- Chenopodium quinoa is a satisfactory local lesion host.
Strains
All isolates found are similar in host range and symptomatology and appear serologically identical.
Transmission by Vectors
Nematode transmission suspected but not established.
Transmission through Seed
No information.
Transmission by Dodder
No information.
Serology
The virus is a good immunogen. Dilution end-points of antisera are up to 1/2048. In gel-diffusion tests with crude sap from systemically infected plums or from herbaceous host plants, the virus gives a good reaction with a single line of precipitation. In immunoelectrophoresis in agarose gels there is only one migrating component.
Relationships
The virus is a member of the nepovirus group. A distant serological relationship was detected to beet ringspot and potato bouquet strains of tomato black ring virus (Delbos et al., 1976). Antiserum to myrobalan latent ringspot virus with a homologous titre of 1/1024 reacts only to 1/4 with the heterologous antigens. No relationship was detected to different strains of the following nepoviruses: cherry leaf roll, arabis mosaic, tobacco ringspot, tomato ringspot, strawberry latent ringspot, raspberry ringspot and artichoke Italian latent.
Stability in Sap
Thermal inactivation point is about 72°C; optimum pH is 7.5 but extracts are infective over a wide range of pH from 2 to 10. Dilution end-point is about 10-4. Longevity at 4°C is more than 3 months.
Purification
Dunez et al. (1971) used either Steere’s butanol-chloroform technique or bentonite treatment. Bentonite is prepared by the method of Dunn & Hitchborn (1965). Leaves are blended in the presence of bentonite (10 mg bentonite for 1 g infected leaves). After filtration, the juice is stored 10 min at 4°C and clarified by centrifugation for 10 min at 3000 rev./min. If the supernatant fluid is still green, further treatments with a small amount of bentonite (2.5 mg/g infected leaves) may be performed. The supernatant fluid after bentonite treatment or the aqueous phase from butanol-chloroform clarification are concentrated by ultracentrifugation and purified by sucrose density gradient centrifugation. Yields of 2.5 mg virus, free of host material, are obtained from 100 g infected leaves.
Properties of Particles
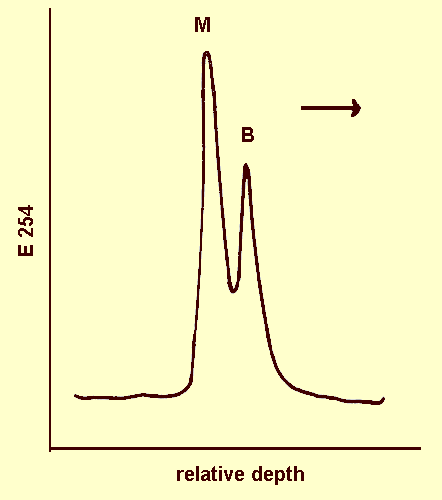
Sucrose gradients show that purified virus preparations contain two sedimenting nucleoprotein components, M and B (Fig. 4) which are serologically identical. The sedimentation rates (s20) of M and B are 105 and 115 S respectively (concentration 100 µg/ml). The relative concentration of B:M is about 0.6.
Isoelectric focusing shows only one component; the iso-electric point of M and B components is about pH 4.5. Electrophoresis of intact virus in 2.6% polyacrylamide gels in 0.01 M Tris, 0.01 M MgCl2, pH 7.0, gives two components.
A260/A280: 1.65 (M), 1.73 (B).
Buoyant density in CsCl: 1.46 (M), 1.50 (B), (Fig. 5).
Particle Structure
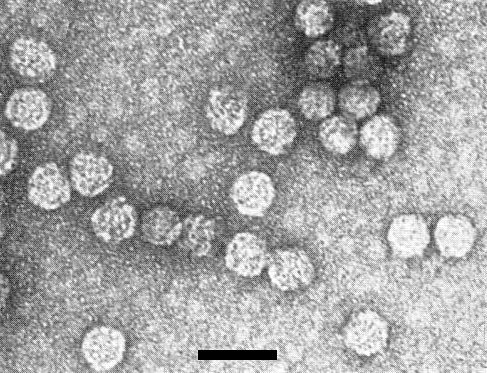
Particles are isometric about 28 nm in diameter (using TMV [18 nm diameter] as a size standard) with rather angular outlines. Some particles are not penetrated by sodium phosphotungstate or uranyl acetate (Fig. 6).
Particle Composition
- Nucleic acid: RNA comprising about 28% (M) or 38% (B) of the
particle weight estimated from ultraviolet absorption spectra. Polyacrylamide
gel electrophoresis of RNA from sodium dodecyl sulphate-treated particles in
2.5% gel shows two major components: RNA-1 (M. Wt 2.6 x 106) and RNA-2 (M. Wt
1.9 x 106) and a minor one RNA-3 (M. Wt 0.45 x 106).
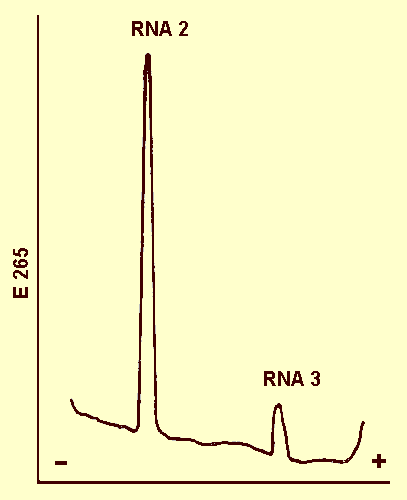
RNA-2 and RNA-3 are present in M particles (Fig. 7); all three RNA species
are present in B particles (Fig. 8).
- Protein: In 7% polyacrylamide gels the protein migrates as one component of M. Wt 53,000.
Relations with Cells and Tissues
No information.
Notes
Its host range, symptoms and several other characteristics, such as number of nucleoproteins, RNA content, number of RNA species and molecular weight of the coat protein, are similar to those observed with tomato black ring virus, to which it is distantly serologically related. However some differences exist, especially in the physical properties of nucleoproteins and RNA. The sedimentation constants of M and B components are closer than with strains of tomato black ring virus and, correlated with this, RNA-2 M. Wt is higher.
Figures
Ultraviolet absorptiometer trace of sucrose density gradient after centrifugation for 2 h at 40,000 rev/min in a Beckman SW40 Rotor.
References list for DPV: Myrobalan latent ringspot virus (160)
- Delbos, Dunez, Barrau & Fisac, Annls Microbiol. A (Institut Pasteur) 127: 101, 1976.
- Dunez, Delbos, Desvignes, Marenaud, Kuszala & Vuittenez, Annls Phytopath. Numéro hors série: 117, 1971.
- Dunn & Hitchborn, Virology 25: 171, 1965.