Details of DPV and References
DPV NO: 161 September 1976
Family: Potyviridae
Genus: Potyvirus
Species: Bidens mottle virus | Acronym: BiMoV
Bidens mottle virus
D. E. Purcifull Plant Virus Laboratory, Plant Pathology Department, University of Florida, Gainesville, Florida 32611, USA
S. R. Christie Plant Virus Laboratory, Plant Pathology Department, University of Florida, Gainesville, Florida 32611, USA
T. A. Zitter Agricultural Research & Education Center, Belle Glade, Florida 33430, USA
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by Christie, Edwardson & Zettler (1968).
- A virus with flexuous, filamentous particles c. 720 nm long. It is transmitted by aphids in the non-persistent manner, is sap-transmissible, and causes mottle diseases of lettuce and endive in Florida, USA.
Main Diseases
Causes mottle diseases of Lactuca sativa (lettuce) and Cichorium endivia (endive and escarole).
Geographical Distribution
Widespread in Florida, USA.
Host Range and Symptomatology
Known to infect 10 species of Compositae and 9 species in 5 other dicotyledonous families. Sap-transmissible.
- Diagnostic species
- Helianthus annuus
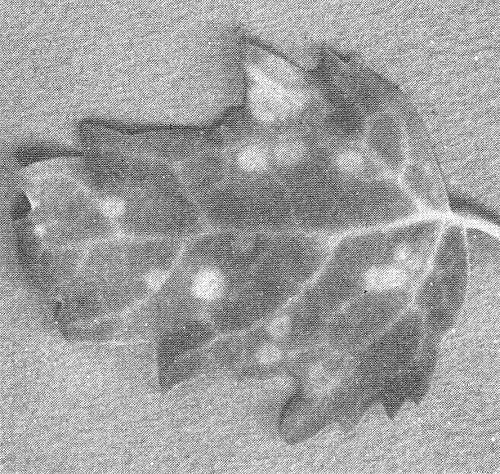
. Faint to bright yellow mottle in systemically infected leaves 5-10 days after inoculation (Fig. 2). - Nicotiana glutinosa x Nicotiana clevelandii hybrid (Christie, 1969). Systemic mottle
about 10 days after inoculation (Fig. 3). Affected plants are stunted and have moderately distorted
leaves.
- Zinnia elegans. Mottle, leaf distortion and stunting.
- Zinnia elegans. Mottle, leaf distortion and stunting.
- Propagation species
- Nicotiana glutinosa x Nicotiana clevelandii
hybrid. Useful for maintaining cultures and as a source of virus for purification.- Assay species
- Chenopodium quinoa
develops chlorotic local lesions in inoculated leaves (Fig. 1), followed by systemic symptoms consisting of chlorotic spots and flecks. - Escarole (Cichorium endivia) is useful as a test plant for aphid transmission.
Strains
Most lettuce cultivars are infected by the type strain (American Type Culture Collection isolate No. PV-165) (Purcifull et al., 1971; Purcifull & Zitter, 1971; Zitter & Guzman, 1974), but the cultivar ‘Valmaine’ is not; a minor natural variant, however, also infects the ‘Valmaine’ cultivar (T. A. Zitter, unpublished data).
Transmission by Vectors
Transmitted in a non-persistent manner by several species of aphids, including Myzus persicae (Christie et al., 1968). Both nymphs and adults of M. persicae can transmit the virus, and this species is probably the most efficient vector (T. A. Zitter, unpublished data).
Transmission through Seed
None detected in Bidens pilosa (S. R. Christie, unpublished data) or in lettuce (T. A. Zitter, unpublished data).
Transmission by Dodder
No information.
Serology
The virus is a good immunogen. It reacts well in immunodiffusion tests conducted in agar gels containing sodium dodecyl sulphate (Purcifull & Zitter, 1973) or in agar gels after pyrrolidine treatment of antigen (Shepard, Secor & Purcifull, 1974). Proteinaceous pinwheel inclusions are also immunogenic, are detectable in sodium dodecyl sulphate-treated plant extracts, and are immunochemically distinct from viral coat protein (Purcifull, Hiebert & McDonald, 1973).
Relationships
The virus has been classified in the potyvirus group on the basis of its particle morphology, its aphid-transmissibility, its ability to induce pinwheel inclusions in its hosts (Christie et al., 1968; Edwardson, 1974), and its serological relationship to potato virus Y (D. E. Purcifull, unpublished data). Immunodiffusion tests with sodium dodecyl sulphate-treated extracts from infected plants indicated that bidens mottle virus is serologically distinct from lettuce mosaic virus (Purcifull & Zitter, 1973) and from turnip mosaic virus (T. A. Zitter & D. E. Purcifull, unpublished data). These three viruses may have some common antigenic determinants, however, because they reacted positively with antiserum to pyrrolidine-degraded tobacco etch virus (Shepard, Secor & Purcifull, 1974).
Stability in Sap
In sap from infected Nicotiana hybrid, the virus was inactivated by heating to 50-55°C for 10 min or by dilution beyond 10-3. The virus retained infectivity after storage for 16 days at 24°C.
Purification
The virus and virus-induced pinwheel inclusions can be purified from the same batch of tissue (Hiebert & McDonald, 1973). Leaves (500 g) from infected Nicotiana hybrid are homogenized in 1 litre of 0.5 M phosphate, pH 7.5, containing 2.5 g sodium sulphite and the homogenate is centrifuged at 9000 g for 10 min. Purify the virus from the supernatant fluid using butanol clarification (8%,v/v), precipitation with polyethylene glycol M. Wt 6000 (8%, w/v), differential centrifugation and equilibrium centrifugation in CsCl. The pellet obtained from the initial centrifugation of the homogenate is resuspended in 0.5 M phosphate containing 0.5% 2-mercaptoethanol, and treated with Triton X-100 (5%, v/v) to disrupt chloroplasts. The inclusions are then further purified by differential centrifugation, filtration, and sucrose density gradient centrifugation.
Properties of Particles
No information.
Particle Structure
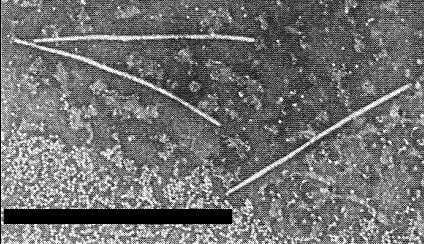
Particles are flexuous filaments (Fig. 4) (Christie et al., 1968), about 720 nm long (Purcifull et al., 1971).
Particle Composition
Nucleic acid: No information.
Protein: Contains a single type of polypeptide subunit, of M. Wt 33,000. Sometimes the subunit is partially degraded to give a component of M. Wt 28,000 (Hiebert & McDonald, 1973).
Relations with Cells and Tissues
The virus induces cytoplasmic pinwheel inclusions and laminated aggregates which can be detected by light microscopy (Fig. 5) and electron microscopy (Fig. 6, Fig. 7) (Christie et al., 1968). The pinwheel inclusion protein subunit has a M. Wt of 69,000 (Hiebert & McDonald, 1973).
Notes
Bidens mottle virus induces symptoms in lettuce and endive that may be confused with those caused by lettuce mosaic and turnip mosaic viruses, which are also potyviruses. These three viruses, however, can be distinguished by serology (Purcifull & Zitter, 1973; Citir & Varney, 1974; T. A. Zitter & D. E. Purcifull, unpublished data) and by reactions in indicator plants. Bidens mottle virus induces mottle symptoms in sunflower and the Nicotiana hybrid, but does not infect pea (Pisum sativum). Conversely, most isolates of lettuce mosaic virus cause mottle symptoms in pea but infect neither sunflower nor the Nicotiana hybrid (Purcifull et al., 1971; Purcifull & Zitter, 1973). Bidens mottle virus failed to induce symptoms in tendergreen mustard (Brassica perviridis) (Purcifull et al., 1971), but most turnip mosaic virus isolates induce a prominent mosaic in this species (McDonald & Hiebert, 1975). Bidens pilosa, a common weed in Florida, is sometimes doubly infected with bidens mottle virus and sonchus yellow net virus, which is an aphid- and sap-transmissible virus with bacilliform particles (Christie, Christie & Edwardson, 1974).
Bidens mottle virus has several properties in common with the bidens mosaic virus reported in Brazil (Kitajima, Carvalho & Costa, 1961). The two viruses have similar particle morphologies, are both aphid transmissible, induce similar types of inclusion bodies in their hosts (E. W. Kitajima, personal communication), and they both infect sunflower, tobacco, Chenopodium amaranticolor, and Bidens pilosa. However, it has not been determined whether they are serologically related.
Figures
References list for DPV: Bidens mottle virus (161)
- Christie, Pl. Dis. Reptr 53: 939, 1969.
- Christie, Christie & Edwardson, Phytopathology 64: 840, 1974.
- Christie, Edwardson & Zettler, Pl. Dis. Reptr 52: 763, 1968.
- Citir & Varney, Proc. Am. Phytopath. Soc. 1: 134, 1974.
- Edwardson, Monograph Ser. Fla agric. Exp. Stn No. 4, 398 pp., 1974.
- Hiebert & McDonald, Virology 56: 349, 1973.
- Kitajima, Carvalho & Costa, Bragantia 20: 503, 1961.
- McDonald & Hiebert, Virology 63: 295, 1975.
- Purcifull, Christie, Zitter & Bassett, Pl. Dis. Reptr 55: 1061, 1971.
- Purcifull, Hiebert & McDonald, Virology 55: 275, 1973.
- Purcifull & Zitter, Proc. Fla St. hort. Soc. 84:165, 1971.
- Purcifull & Zitter, Proc. Fla St. hort. Soc. 86: 143, 1973.
- Shepard, Secor & Purcifull, Virology 58: 464, 1974.
- Zitter & Guzman, Pl. Dis. Reptr 58: 1087, 1974.