Details of DPV and References
DPV NO: 164 September 1976
Family: Bromoviridae
Genus: Ilarvirus
Species: Citrus leaf rugose virus | Acronym: CiLRV
Citrus leaf rugose virus
S. M. Garnsey U.S. Horticultural Research Laboratory, ARS, USDA, Orlando, Florida 32803, USA
D. Gonsalves Institute of Food and Agricultural Sciences, University of Florida, Agricultural Research and Education Center, Lake Alfred, Florida 33850, USA
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by Garnsey (1975).
Synonyms
- Citrus crinkly-leaf-type virus (Rev. Pl. Path. 49: 330c)
An RNA-containing virus with isometric particles 25 to 32 nm in diameter, which sediment as four nucleoprotein components. Readily sap-transmissible to many citrus and herbaceous hosts. Vector unknown, reported only from Florida.
Main Diseases
Causes leaf flecking in Eureka lemon (Citrus limon), leaf rugosity in Mexican lime (C. aurantifolia), and severe stunting of young Duncan grapefruit (C. paradisi) plants (Garnsey, 1968).
Geographical Distribution
Reported only from one location in Florida (Garnsey, 1968; Garnsey & Purcifull, 1969).
Host Range and Symptomatology
Restricted in nature to citrus but can be transmitted mechanically to a wide range of herbaceous plants (Garnsey, 1975).
-
Diagnostic species
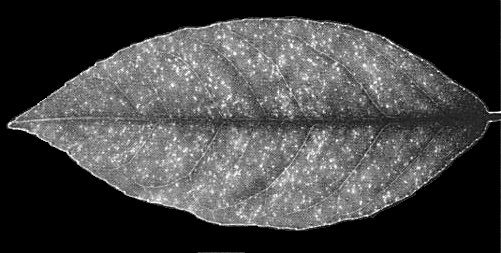
- C. aurantifolia
(Mexican lime). Variable rugosity of leaves (Fig. 1). - C. limon (Eureka lemon). Pinpoint chlorotic flecks on expanding leaves,
persistent without leaf distortion
(Fig. 2).
- C. paradisi (Duncan grapefruit). Severe stunting and chlorosis (some isolates).
- Phaseolus vulgaris (bean) cv. Red Kidney. Small, necrotic local lesions (Fig. 3). Not systemic.
- C. paradisi (Duncan grapefruit). Severe stunting and chlorosis (some isolates).
-
Propagation species
- Etrog citron (C. medica) and most other citrus varieties are suitable for maintaining cultures. Tender new growth is a good source for purification. Gomphrena globosa, Nicotiana tabacum and Petunia hybrida can also be used.
-
Assay species
- Phaseolus vulgaris
cv. ‘Red Kidney’, Vigna unguiculata (Early Ramshorn cowpea), and Crotalaria spectabilis can be used as local lesion hosts. Other cvs. of bean and cowpea are also local lesion hosts (Garnsey, 1975).
Strains
Two strains, which differ in their effect on Duncan grapefruit, have been described (Garnsey, 1975). A culture has been submitted to the American type culture collection as PV195.
Transmission by Vectors
Vectors unknown, but field spread is suspected (Garnsey, 1968). The virus has been experimentally transmitted as a contaminant on cutting tools.
Transmission through Seed
None reported. Nucellar seedlings from apparently naturally infected seedling trees of Orlando tangelo (C. paradisi x C. reticulata) were free of the virus (Garnsey, 1975).
Transmission by Dodder
Not tested.
Serology
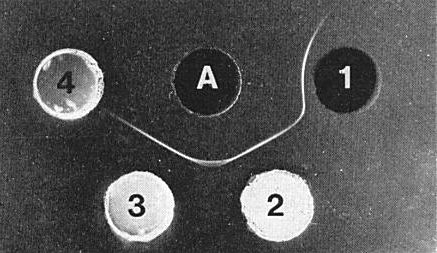
The virus is moderately immunogenic. Antisera with titres of 1/256 to 1/512 in gel diffusion tests have been obtained. Gels containing 0.75% agar and 0.04% sodium azide are preferable to gels with NaCl (8.5%) or phosphate buffer (0.05 M) added. A single precipitin band which curves to the antigen well (Fig. 6) forms in gel-diffusion tests with purified virus preparations containing all four components. Extracts from infected plants or purified virus mixed with sap from healthy plants contain a single, faster-moving antigen that forms a straight precipitin line when allowed to react in gel-diffusion plates with antisera prepared against the virus. No spur forms at the junction of precipitin lines formed to fast and slow antigens (Fig. 6). The virus can easily be detected serologically in extracts from young citrus leaves (Garnsey & Purcifull, 1969).
Relationships
Two strains distinguished by reaction in Duncan grapefruit (Garnsey, 1975) are serologically indistinguishable. The virus is serologically related to citrus variegation virus and citrus crinkly leaf virus; however, spur formation in gel diffusion plates and a 4-fold or greater difference in homologous and heterologous titres clearly show the viruses are not identical (Garnsey, 1974; 1975). Recently, a similar serological relationship to Tulare apple mosaic virus has been found (R. M. Lister, D. Gonsalves & S. M. Garnsey, unpublished data). Tests for serological relationships to tobacco streak, cowpea mosaic and alfalfa mosaic viruses were all negative (Garnsey, 1975; Gonsalves & Garnsey, 1975c). Properties, structure and composition of the particles place citrus leaf rugose virus in the ilarvirus group.
Cross protection between the two described strains of citrus leaf rugose virus occurs in Duncan grapefruit plants (Garnsey, 1975). The virus affords at least partial protection in Eureka lemon and Etrog citron plants against challenge inoculation by citrus variegation virus (Garnsey, 1975). Citrus variegation virus also protects Red Kidney bean plants against challenge inoculation with citrus leaf rugose virus (D. Gonsalves & S. M. Garnsey, unpublished data).
Stability in Sap
The thermal inactivation point in citrus leaf extracts prepared in 0.05 M phosphate buffer or of purified preparations is between 60 and 65°C. Extracts of young citrus leaves prepared in phosphate buffer retain some infectivity for 48 h at room temperature and remain highly infective for 48 h at 4°C (Garnsey, 1975).
Purification
Virus can be purified from young, infected citrus or tobacco leaves by the following method (Garnsey, 1975). Grind leaves in pH 7.4 buffer (1 g/3 ml) containing 0.02 M potassium phosphate, 0.01 M sodium diethydithiocarbamate, and 0.02 M sodium thioglycollate. Squeeze extract through cheesecloth. Mix filtrate thoroughly with 0.7 ml hydrated calcium phosphate gel/g tissue, and clarify by low speed centrifugation. Subject supernatant fluid to two cycles of differential centrifugation and resuspend final high-speed pellets in pH 7.2 buffer containing 0.005 M potassium phosphate and 0.005 M MgCl2. The virus is further purified by rate-zonal density-gradient centrifugation in sucrose gradients. Virus yields from citrus leaves are about 3-5 mg/100 g tissue. Yields from tobacco leaves are about 1-2 mg/100 g tissue.
Properties of Particles
As shown in Fig. 4, purified preparations contain four nucleoprotein components (Garnsey, 1975). These are named according to increasing sedimentation velocities as: NP 4, NP 3, NP 2, and NP 1, respectively (Gonsalves & Garnsey, 1975b). Purified NP 3 and NP 4 are not infective alone or in combination (Gonsalves & Garnsey, 1974). Preparations of NP 1 +NP 2 are moderately infective and become highly infective with the addition of NP 3. The relative amount of the different components is similar, regardless of the host in which the virus was propagated.
Sedimentation coefficients (s20, w) (svedbergs): 79 (NP 4), 89 (NP 3), 98 (NP 2), and about 105 (NP 1). Calculations not made for infinite dilution.
Isoelectric point: between pH 4.4 and 5.0.
Absorbance at 260 nm (1 mg/ml, 1 cm light path): 5.3 for preparations containing all four components.
A260/A280: Ratios of 1.40 to 1.46 were found for purified preparations containing all four components. Similar ratios were found for NP 3 and for a mixture of NP 1 and NP 2.
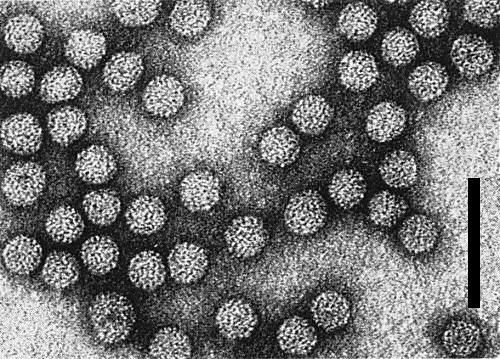
Particle Structure
Particles are essentially isometric, with some heterogeneity in size (Fig. 5). Particle diameter correlates positively with the sedimentation rate of the components: c. 24.8 nm (NP 4), c. 26.3 nm (NP 3), c. 31.3 nm (NP 2), and c. 32.2 nm (NP 1) (Gonsalves & Garnsey, 1976). Particles are partially disrupted in phosphotungstic acid unless fixed in formaldehyde.
Particle Composition
Nucleic acid: RNA, single-stranded. The amount of RNA in the particles has not been accurately determined, but based on A260/A280 ratios is estimated to be similar to that of Tulare apple mosaic and tobacco streak viruses (12-14%). The RNAs in NPs 1, 2, 3 and 4 are designated as RNAs 1, 2, 3 and 4, respectively (Gonsalves & Garnsey, 1974). Their M. Wts are: c. 1.1 x 106, c. 1.0 x 106, c. 0.7 x 106, and c. 0.3 x 106, respectively. Sedimentation data indicate that two molecules of RNA 4 are probably enclosed in one NP 4 particle. Very small amounts of a fifth RNA (RNA 4a) are usually observed in gels. The RNAs are not infective singly. However, non-infective mixture of RNAs 1+2+3 became highly infective with the addition of either RNA 4 or coat protein (Gonsalves & Garney, 1975a). Coat protein and RNA-4 of citrus variegation virus or alfalfa mosaic virus, and coat protein from tobacco streak virus are also effective (Gonsalves & Garnsey, 1975c). RNA 4 or coat protein from citrus leaf rugose virus will activate non-infective mixtures of RNAs 1+2+3 from alfalfa mosaic virus, and from citrus variegation virus.
Protein: A single polypeptide species with M. Wt of c. 26,000 based on SDS-polyacrylamide gel-electrophoresis.
Relations with Cells and Tissues
The virus is present in most plant tissues. Cellular associations, and occurrence of inclusions have not been investigated.
Notes
The virus has many properties similar to those of citrus variegation and Tulare apple mosaic viruses. However, although these viruses are related, they are easily distinguished by biological properties and serological differences. Citrus variegation virus does not produce rugose symptoms in Mexican lime, and citrus leaf rugose virus does not cause severe distortion and variegation patterns in Etrog citron. Citus leaf rugose virus causes only local necrotic symptoms in the bean and cowpea varieties tested, whereas citrus variegation virus commonly causes systemic symptoms. Sedimentation profiles for the nucleoprotein components of the two viruses are different, with citrus leaf rugose virus showing more NP 4 and poorer separation of NP 1 and NP 2 (Gonsalves & Garnsey, 1975b). Recognizable differences in the nucleic acid species of these two viruses also exist (Gonsalves & Garnsey, 1975b). Similarly, citrus leaf rugose virus differs in biological and physical properties from Tulare apple mosaic virus. The latter causes chlorotic local lesions in cucumber (Cucumis sativus), vein banding in Gomphrena globosa and necrotic local and shock symptoms in tobacco, none of which is caused by citrus leaf rugose virus. Tulare apple mosaic virus is less stable, and the sedimentation profile of its nucleoprotein components is different.
Figures

(Top) Ultraviolet absorbance profile of virus nucleoprotein centrifuged 14 h in 20-50% sucrose gradient at 113,000 g. (Bottom) RNA preparation electrophoresed in 2.5% polyacrylamide gel. Arrows indicate direction of sedimentation or migration.
References list for DPV: Citrus leaf rugose virus (164)
- Garnsey, Proc. Fla St. hort. Soc. 81: 79, 1968.
- Garnsey, Proc. 6th Conf. int. Organisation of Citrus Virologists 1974: 169, 1974.
- Garnsey, Phytopathology 65: 50, 1975.
- Garnsey & Purcifull, Proc. Fla St. hort. Soc. 81: 56, 1969.
- Gonsalves & Garnsey, Virology 61: 343, 1974.
- Gonsalves & Garnsey, Virology 64: 23, 1975a.
- Gonsalves & Garnsey, Virology 67: 311, 1975b.
- Gonsalves & Garnsey, Virology 67: 319, 1975c.
- Gonsalves & Garnsey, Proc. 7th Conf. int. Organisation of Citrus Virologists, 1976: 109, 1976.