Details of DPV and References
DPV NO: 17 June 1970
Family: Secoviridae
Genus: Nepovirus
Species: Tobacco ringspot virus | Acronym: TRSV
There is a more recent description of this virus: DPV 309
Tobacco ringspot virus
R. Stace-Smith Canada Agriculture Research Station, Vancouver 8, B.C., Canada
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by
Fromme, Wingard & Priode (1927).
- Selected synonyms
- Tobacco ringspot virus No. 1 (Rev. appl. Mycol. 15: 831)
- Annulus tabaci (Rev. appl. Mycol. 28: 514)
- Nicotiana virus 12 (Rev. appl. Mycol. 36: 303)
- Annulus tabaci (Rev. appl. Mycol. 28: 514)
- An RNA-containing virus with isometric particles about 28 nm in diameter. It is readily transmitted by sap inoculation and has a wide host range, including both herbaceous and woody plants. Transmitted by the nematode Xiphinema americanum, and also by species of thrips, spider mite, grasshopper, flea beetle and, possibly, aphid. Commonly seed transmitted.
Main Diseases
Causes ringspot diseases of tobacco, cucumber, Easter lily, hydrangea, iris and Pelargonium; also blueberry necrotic ringspot, soybean bud blight, and chlorotic or necrotic spotting in many other annual and perennial crops.
Geographical Distribution
Occurs in the USA and Canada, field spread being confined primarily to areas where Xiphinema americanum is prevalent. The virus has been distributed, probably in ornamental crops, to other parts of the world, including the UK, Germany and Australia.
Host Range and Symptomatology
Host range wide; has been found infecting, or transmitted experimentally
to, plants of at least 38 genera, representing 17 families
(Price, 1940).
The
anemone necrosis strain infected 58 of 103 plant spp. tested, in 21 of 32
families
(Hollings, 1965).
The nematode vector, Xiphinema americanum,
can transmit the virus to roots of Cupressus arizonica
(Fulton, 1969).
Diagnostic species
- Cucumis sativus (cucumber;
Fig. 1). Chlorotic local lesions, systemic
mottling and dwarfing, severe apical distortion.
- Nicotiana tabacum (tobacco; Fig. 3), N. glutinosa and N. clevelandii (Fig. 5). Necrotic local lesions that frequently develop into rings or ringspots; systemic ring or line patterns. Leaves produced later show no symptoms but contain virus.
- Lycopersicon esculentum (tomato). Difficult to infect by sap inoculation; plants that are infected develop small necrotic spots.
- Phaseolus vulgaris (bean). Necrotic spots on inoculated leaves; systemically infected leaves show spots and rings, and the growing tip becomes necrotic.
- Vigna sinensis (cowpea; Fig. 2). Necrotic local lesions, systemic necrosis, apical necrosis and wilt. Cowpea varieties may be used for distinguishing strains.
- Chenopodium amaranticolor and C. quinoa. Local necrotic dots; usually no systemic reaction.
- Nicotiana tabacum (tobacco; Fig. 3), N. glutinosa and N. clevelandii (Fig. 5). Necrotic local lesions that frequently develop into rings or ringspots; systemic ring or line patterns. Leaves produced later show no symptoms but contain virus.
- Propagation species
- Cucumber or tobacco are suitable hosts for maintaining cultures. Cucumber
or bean are good sources of virus for purification.
- Assay species
- Vigna sinensis, Nicotiana tabacum, N. clevelandii and Cassia occidentalis are useful local lesion hosts. Cucumber is a useful source and bait plant for nematode transmission experiments (Fulton, 1962).
Strains
Many minor variants can be distinguished. The best known major variants are:
Tobacco green ringspot strain of
Valleau (1932).
Produces necrotic
and pale yellow lesions in tobacco; recovered leaves are green. Local lesions
in cowpea are necrotic at edges only. Source USA.
Tobacco yellow ringspot strain of
Valleau (1932).
Produces bright
yellow as well as necrotic local lesions in tobacco; recovered leaves are yellow.
Local lesions in cowpea are necrotic at edges only. Source USA.
Tobacco ringspot virus No. 1 of
Price (1936).
Produces necrotic and
pale yellow lesions in tobacco, causes more necrosis and stunting than tobacco
green ringspot virus; recovered leaves are green. Produces solid necrotic
spots in cowpea. Source USA.
Eucharis mottle strain of
Kahn, Scott & Monroe (1962). Produces
necrotic or chlorotic rings in some tobacco varieties but is latent in others.
Ringspot lesions followed by recovery in Gomphrena globosa. Source Peru.
Anemone necrosis strain of
Hollings (1965).
Produces local necrotic
spots and rings in tobacco; chronic symptoms are chlorosis, vein-banding and
dwarfing. Produces necrotic local lesions in cowpea, followed by apical necrosis
and death of plant. Source UK.
Satellite-like strain of Schneider (1969). Non-infective alone but multiplies in bean and cowpea when mixed with a tobacco isolate. Produces small dark local lesions, usually surrounded by a clear zone, in inoculated leaves of cowpea.
Transmission by Vectors
Transmitted by adults and three larval stages of the nematode Xiphinema americanum. Single nematodes may transmit (McGuire, 1964). Nematodes can acquire virus within 24 h. Infective nematodes stored at 10°C for 49 weeks could still transmit (Bergeson et al., 1964).
The virus is also transmitted in soybean by nymphs but not adults of Thrips tabaci (Messieha, 1969). They acquire virus within 8 hr and remain infective for 14 days.
The virus is transmitted less efficiently by spider mites of the genus Tetranychus (Thomas, 1969), grasshoppers of the genus Melanoplus (Dunleavy, 1957) and the tobacco flea beetle, Epitrix hirtipennis (Schuster, 1963). There are also reports of transmission by the aphids Myzus persicae and Aphis gossypii (Komuro & Iwaki, 1968; Rani, Verma & Verma, 1969), but the aphid-transmitted virus in begonia (Semal, 1958) is probably not tobacco ringspot virus.
Transmission through Seed
Common in soybean, petunia, Nicotiana glutinosa, Gomphrena globosa and Taraxacum officinale; rare in tobacco, cantaloupe, cucumber, muskmelon and lettuce. Pollen transmission not investigated.
Transmission by Dodder
Not transmitted by Cuscuta campestris (Bennett, 1944).
Serology
The virus is moderately immunogenic. Antiserum titres in excess of 1:2000 are readily attained. Diagnostic serological reactions can be obtained in ring precipitin, microprecipitin, tube precipitin, or gel-diffusion tests. Antisera prepared by intravenous or intramuscular injections give a single band of precipitate in gel-diffusion tests. Agar gel tests on microscope slides are not always satisfactory (Hollings, 1965).
Relationships
The Eucharis mottle strain is serologically related but not identical to the tobacco isolates, as shown by spur formation in agar gel-diffusion tests (Kahn et al., 1962). Four distinct serological strains have been detected in tobacco (Gooding, 1969). Also, a strain isolated from trailing blackberry is antigenically related to, but not identical with, an isolate from tobacco (Rush, Gooding & Ellis, 1968).
In plant-protection tests, recovered tobacco plants are partially or completely protected against invasion by related strains, Gomphrena globosa plants that have recovered from infection with the type isolate from tobacco, are protected against invasion by the Eucharis mottle strain but, in reciprocal tests, plants that have recovered from infection with the Eucharis mottle strain are not protected against invasion by the tobacco strain (Kahn et al., 1962).
Stability in Sap
In petunia sap, diluted 1:5 with distilled water, the thermal inactivation point (10 min) for most isolates is 65°C, although a blueberry isolate is inactivated at 55°C (Lister, Raniere & Varney, 1963). The dilution end point of most isolates is c. l0-4. Sap is infective after 6-10 days at room temperature, 3 weeks at 18°C, and several months at 2°C. Lyophilized sap containing the anemone necrosis isolate was infective after 5 years in sealed ampoules (Hollings, 1965).
Purification
The virus is relatively stable and many purification techniques are satisfactory. Squash or cucumber seedlings or inoculated leaves of cowpea may yield 50-100 mg virus per kg tissue.
The widely used butanol-chloroform procedure was developed for the purification of this virus (Steere, 1956). It gives satisfactory yields of most strains, but extracts containing the anemone necrosis strain are best clarified by adding n-butanol to 8.5% (v/v) (Hollings, 1965). Other techniques that are rapid and give good yields involve removing impurities with activated charcoal followed by density gradient centrifugation (Corbett & Roberts, 1962) or addition of ammonium sulphate (200 g/l clarified sap), which removes host proteins with little loss of virus (Stace-Smith, Reichmann & Wright, 1965).
Properties of Particles
Purified preparations contain three major classes of particles
(Fig. 6),
empty protein shells without RNA (T), non-infectious nucleoprotein (M), and
infectious nucleoprotein (B)
(Stace-Smith et al., 1965).
Also recognized
is a satellite-like nucleoprotein (SL) which is non-infective alone but infective
when mixed with the multi-component virus
(Schneider, 1969).
All particles are
serologically indistinguishable.
Sedimentation coefficients (s20,w) at infinite dilution (svedbergs): 53 (T), 91 (M), 126 (B) and 122 (SL).
Molecular weights (daltons): 3.3 x 106 (T), 4.9 x 106 (M), 5.7 x 106 (B).
Diffusion coefficient (D20 x 10-7 cm2/sec): 1.59 (B).
Isoelectric point: between pH 4.2 and 6.1 (B).
Electrophoretic mobility: -1.39 x l0-5 cm2 volt-1 sec-1 in 0.1 ionic strength phosphate, pH 6.5.
Absorbance at 260 nm (1 mg/ml, 1 cm light path): about 10.0 (B).
A260/A280: 0.91 (T), 1.37 (M), 1.88 (B).
Particle Structure
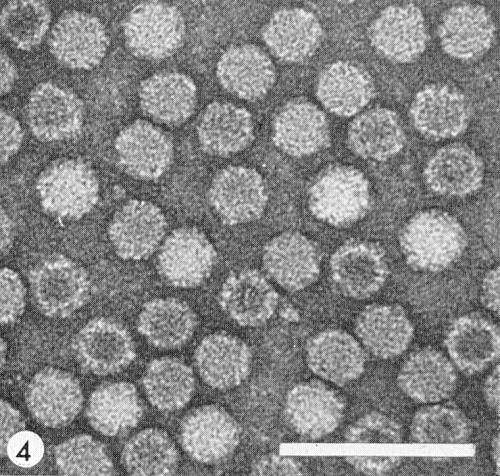
Particles are isometric, about 29 nm in diameter (Fig. 4), and have 42 morphological subunits (Chambers, Francki & Randles, 1965). Particles are stable when mounted in phosphotungstate or uranyl acetate for electron microscopy. Particles penetrated by negative stain do not necessarily represent particles devoid of RNA; the proportion of particles penetrated increases with increasing pH of the stain but staining time has no effect (Davison & Francki, 1969).
Particle Composition
RNA: The 126 S nucleoprotein particle may contain one infectious or two non-infectious nucleic acid strands per particle (Diener & Schneider, 1966). The 91 S particle contains one non-infectious strand. The infectious strand has a molecular weight of 2.2 x 106 and a sedimentation coefficient of c. 32 S. The non-infectious strand has a molecular weight of 1.2 x 106 and a sedimentation coefficient of c. 24 S. With increasing age of infection the proportion of 126 S particles with two non-infectious strands increases (Schneider & Diener, 1968). The RNA of the SL nucleoprotein has a molecular weight of 86,000 and a sedimentation coefficient of 7 S. The SL particle is thought to contain 14 or more RNA strands of about the same size (Schneider, 1969).
Protein: About 60% of the particle weight. No evidence of more than one protein species. For amino acid composition, see Stace-Smith et al. (1965).
Relations with Cells and Tissues
No crystalline aggregates of virus particles were seen in root tip cells of beans or leaf cells of bean, cucumber or cowpea. The virus was seen in the terminal 0.5 mm of the root tips of infected beans - files of virus particles occurred in tubules that passed through plasmodesmata and into the cytoplasm of neighbouring cells (Crowley et al., 1969).
Notes
The most damaging disease caused by tobacco ringspot virus is bud blight of
soybean, and the importance of the various modes of transmission in this crop
is not resolved. Seed-transmission occurs but is not considered important in
major periodic outbreaks. Transmission by the nematode X. americanum
is considered negligible, primarily because the virus does not move readily to
the foliage of plants infected through the roots. Aerial vectors are suspected
and of these, thrips, mites, grasshoppers and beetles have been incriminated.
The geographical distribution, natural host range and vector relationships of the virus are similar to those of tomato ringspot virus. Differential host reactions may be useful in distinguishing isolates of the two viruses (McLean, 1962) but serological tests are essential for positive identification. The virus is also similar in size, shape, physical properties and host reactions to several other nepoviruses, but is not serologically related to any of them.
Figures
References list for DPV: Tobacco ringspot virus (17)
- Bennett, Phytopathology 44: 905, 1944.
- Bergeson, Athow, Laviolette & Thomasine, Phytopathology 54: 723, 1964.
- Chambers, Francki & Randles, Virology 25: 15, 1965.
- Corbett & Roberts, Phytopathology 52: 902, 1962.
- Crowley, Davison, Francki & Owusu, Virology 39: 322, 1969.
- Davison & Francki, Virology 39: 235, 1969.
- Diener & Schneider, Virology 29: 100, 1966.
- Dunleavy, Phytopathology 47: 681, 1957.
- Fromme, Wingard & Priode, Phytopathology 17: 321, 1927.
- Fulton, Phytopathology 52: 375, 1962.
- Fulton, Phytopathology 59: 236, 1969.
- Gooding, Phytopathology 59: 114, 1969.
- Hollings, Ann. appl. Biol. 55: 447, 1965.
- Kahn, Scott & Monroe, Phytopathology 52: 1211,1962.
- Komuro & Iwaki, Ann. phytopath. Soc. Japan 34: 7, 1968.
- Lister, Raniere & Varney, Phytopathology 53: 1031, 1963.
- McGuire, Phytopathology 54: 799, 1964.
- McLean, Pl. Dis. Reptr 46: 877, 1962.
- Messieha, Phytopathology 59: 943, 1969.
- Price, Phytopathology 26: 665, 1936.
- Price, Am. J. Bot. 27: 530, 1940.
- Rani, Verma&Verma, Pl. Dis. Reptr 53: 903, 1969.
- Rush, Gooding & Ellis, Phytopathology 58: 1065, 1968.
- Schneider, Science, N.Y. 166: 1627,1969.
- Schneider & Diener, Virology 35: 150, 1968.
- Schuster, Pl. Dis. Reptr 47: 510, 1963.
- Semal, Nature, Lond. 182: 1688, 1958.
- Stace-Smith, Reichmann & Wright, Virology 25: 487, 1965.
- Steere, Phytopathology 46: 60, 1956.
- Thomas, Phytopathology 59: 633, 1969.
- Valleau, Bull. Ky agric. Exp. Stn. 327: 43, 1932.