Details of DPV and References
DPV NO: 173 September 1977
Family:
Genus:
Species: | Acronym:
Cacao necrosis virus
R. H. Kenten Rothamsted Experimental Station, Harpenden, Hertfordshire, England
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Disease described by Posnette (1948) and Thresh (1958);
virus partially characterized by Kenten (1972).
- Synonym
- Cacao swollen shoot virus, strain S (IS, Asalu) (Posnette, 1950).
- A virus with isometric particles 24-26 nm in diameter, infecting cocoa in West Africa. It is readily transmitted by inoculation of sap, has a wide host range and is seed transmitted in some legumes.
Main Diseases
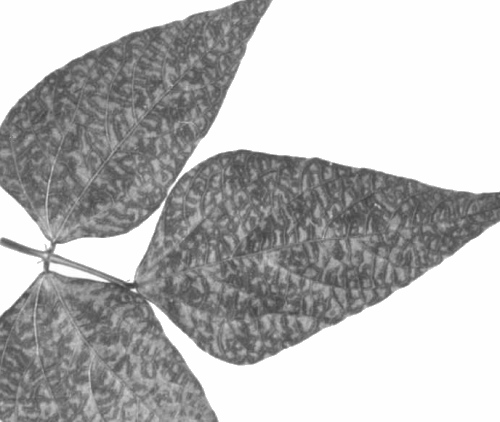
Causes leaf necrosis, defoliation and dieback of cocoa (Theobroma cacao); young seedlings may be killed by the Ghanaian isolate. Plants that survive the acute phase of infection show some leaves with irregular transparent or translucent lesions alongside the major veins (Fig. 1) (Thresh & Tinsley, 1959; Owusu, 1971).
Geographical Distribution
Restricted to W. Africa, infecting cocoa growing at several localities around Asalu in W. Nigeria and in a few small areas in the Brong-Ahafo and Ashanti Regions of Ghana (Thresh, 1958; Owusu, 1971; Legg, Asare-Nyako & Lovi, 1973).
Host Range and Symptomatology
Restricted in nature to cocoa but Kenten (1972) transmitted the Ghanaian isolate by sap inoculation to 15 species in 8 dicotyledonous families.
- Diagnostic species
- Phaseolus vulgaris (French bean) cv. The Prince.
Inoculated primary leaves develop small circular chlorotic
lesions or chlorotic rings 4-5 days after inoculation
(Fig. 4); subsequent trifoliolate leaves show a pronounced
chlorotic mottle often in the form of vein-banding (Fig. 2).
- Beta vulgaris (beet) cv. Greentop. Red ring lesions in inoculated leaves 10 days after inoculation (Fig. 3). No systemic infection.
- Chenopodium quinoa. Severe tip necrosis 10-12 days after inoculation.
- Beta vulgaris (beet) cv. Greentop. Red ring lesions in inoculated leaves 10 days after inoculation (Fig. 3). No systemic infection.
- Propagation species
- P. vulgaris cv. The Prince. Useful for
maintaining cultures and as a source of virus for
purification.
- Assay species
- P. vulgaris cv. The Prince.
Strains
The only isolate from Ghana studied in detail is distinguished from Nigerian isolates by its greater virulence. Graft inoculation of Amelonado cocoa seedlings with the Ghana isolate always causes a severe tip necrosis and subsequently most of the seedlings die. Nigerian isolates are rarely lethal and do not readily induce necrotic symptoms unless the test seedlings are coppiced immediately after grafting (Martini, 1960; Owusu, 1971). It is not known whether Ghanaian and Nigerian isolates are related serologically.
Transmission by Vectors
Not transmitted by the aphids, mealybugs, beetles or leafhoppers that commonly infest cocoa. Attempts both in Ghana and Nigeria to demonstrate soil transmission failed (Martini, 1960; Owusu & Kenten, 1972).
Transmission through Seed
1-24% of seeds from P. vulgaris cv. The Prince infected with the Ghanaian isolate gave rise to infected, but symptomless, plants (Kenten, 1972). The virus is also transmitted through seed of Phaseolus lunatus and Glycine max, but not cocoa (G. K. Owusu, unpublished data).
Serology
The Ghanaian isolate is moderately immunogenic giving antisera with titres of up to 1/512 in rabbits following two intravenous injections. Antisera react with homologous antigen to give granular precipitates in tube precipitin tests and a single line in gel double diffusion tests.
Relationships
The Ghanaian isolate is distantly serologically related to tomato black ring virus and very distantly to grapevine chrome mosaic virus. Absorption tests suggest that it may be considered a serotype, rather than a strain of tomato black ring virus (Kenten, 1972).
Stability in Sap
Sap from P. vulgaris containing the Ghanaian isolate was infective after 10 min at 60°C but not 65°C, after dilution to 10-2 and occasionally 10-3 but not 10-4 and after 4 but not 7 days at 20-24°C. When stored over carbon tetrachloride, sap was still infective after 1 month at 4°C. Sap freeze-dried with 7% peptone and 7% dextrose was still infective after 5 years in vacuo (Kenten, 1972).
Purification
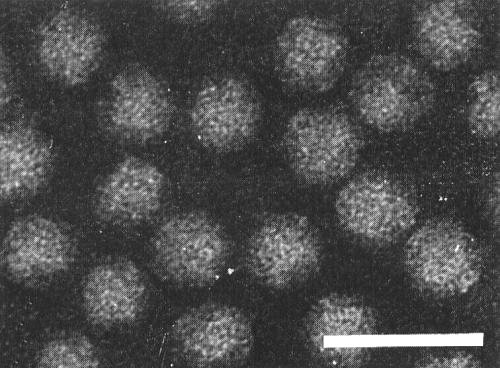
Kenten (1972): harvest inoculated and systemically infected P. vulgaris leaves 12-16 days after infection and triturate in an equal weight of 0.1 M phosphate buffer at pH 7.5 containing 0.05 M ethylenediamine-tetraacetate and 0.02 M thioglycollate (P-E-T). Separate the fluid from the leaf debris by squeezing through cloth and add n-butanol to 8.5% (v/v). After 1 h clarify at 10,000 g for 20 min and sediment the virus at 75,000 g for 2 h. Disperse the virus in a small volume of P-E-T, clarify by low speed centrifugation and then precipitate it by adding 70 g polyethylene glycol (PEG) (M. Wt 6000) and 25 g NaCl per litre and stirring for 1 h at 4°C. Collect the precipitate by centrifuging, further purify and concentrate the virus by three or four cycles of differential centrifugation from 0.02 M phosphate buffer, pH 7. Such preparations contain both nucleoprotein components (Fig. 5, Fig. 7). Yield 50-100 A1 cm,260 units/kg leaf.
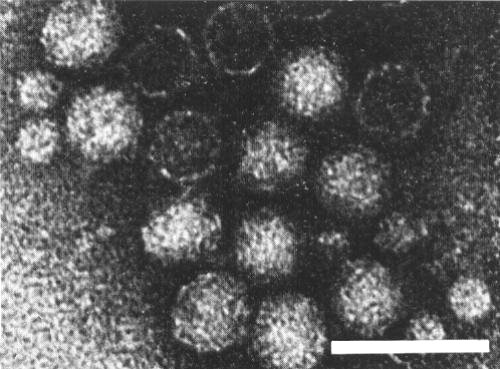
If the leaf extract is not clarified with butanol, but the virus precipitated first with ammonium sulphate and then with PEG followed by differential centrifugation, the preparations contain empty protein shells and a minor component consisting of small spherical particles 12 nm in diameter, in addition to the two nucleoprotein components (Fig. 6, Fig. 8) (Kenten, 1972).
Properties of Particles
There are three major classes of particles, empty protein shells (T), noninfective nucleoprotein (M) and infective nucleoprotein (B). The minor component is polydisperse and is believed to consist of polymorphic forms of coat protein. The empty protein shells are disrupted and precipitated by n-butanol which explains why they are rarely seen in preparations made using n-butanol.
Sedimentation coefficients, s20,w (svedbergs): 20-30 (minor component); 54 (T); 101 (M); 129 (B).
A260/A280: 1.63 (M); 1.78 (B). A260/A240: 1.40 (M); 1.58 (B).
All values corrected for light-scattering.
Particle Structure
The (T), (M) and (B) particles all measure 24-26 nm and have a hexagonal profile when mounted in phosphotungstate or uranyl acetate. The weight of the protein subunit and the nucleic acid content is similar to that of other nepoviruses; the particles probably have an icosahedral structure of 60 structural subunits, each consisting of a single polypeptide molecule.
Particle Composition
Nucleic acid: approximately 30% (M) and 41% (B) of particle weight calculated from sedimentation coefficients, or 22% (M) and 35% (B) calculated from the A260/A280 ratio.
Protein: a single species of polypeptide, M. Wt 60,000, is obtained by disrupting a mixture of the two nucleoprotein components and electrophoresis in acrylamide/SDS gels.
Relations with Cells and Tissues
No information.
Notes
Although two attempts to transmit the virus through soil failed, its properties and serological relationship to tomato black ring virus suggest that a nematode is the natural vector. Longidorus spp. have been found in the soils associated with two outbreaks of cocoa necrosis disease in Ghana.
When healthy Amelonado cocoa seedlings are grafted with bark patches from healthy cocoa or cocoa infected with cacao swollen shoot, cacao mottle leaf or cacao yellow mosaic viruses, 95% or more of the patches form unions. If the grafts are from cocoa infected with the Ghana isolate of cacao necrosis virus, about 90% of the grafts fail to unite; nevertheless, some 80% of the test seedlings are infected (Owusu & Kenten, 1972). Possibly, the bark patches start to unite with the stock and virus is transmitted but a necrotic reaction follows and the grafts fail.
Figures

Translucent lesions produced in a cocoa leaf in the chronic stage of infection. (Photo courtesy J. M. Thresh, East MaIling Research Station).
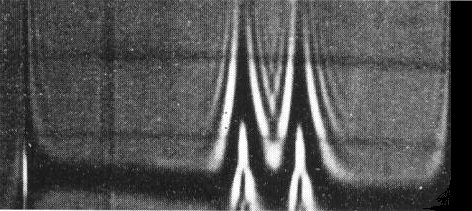
Schlieren pattern produced by cocoa necrosis virus after centrifugation for 12 min at 29,500 rev/min; schlieren angle 40°; sedimentation from left to right.Preparation purified by treatment with n-butanol.

Schlieren pattern produced by cocoa necrosis virus after centrifugation for 12 min at 29,500 rev/min; schlieren angle 40°; sedimentation from left to right. Preparation made using ammonium sulphate instead of n-butanol.
References list for DPV: Cacao necrosis virus (173)
- Kenten, Ann. appl. Biol. 71: 119, 1972.
- Legg, Asare-Nyako & Lovi, Rep. Cocoa Res. Inst. Ghana 1971-72: 73, 1973.
- Martini, Rep. W. Afr. Cocoa Res. Inst. 1958-59: 67, 1960.
- Owusu, Trop. Agric. Trin. 48: 133, 1971.
- Owusu & Kenten, Rep. Cocoa Res. Inst. Ghana 1969-70: 61, 1972.
- Posnette, Rep. W. Afr. Cocoa Res. Inst. 1947-48: 11, 1948.
- Posnette, Ann. appl. Biol. 37: 378, 1950.
- Thresh, Rep. W. Afr. Cocoa Res. Inst. 1956-57: 72, 1958.
- Thresh & Tinsley, Tech. Bull. W. Afr. Cocoa Res. Inst. 7: 8 pp., 1959.