Details of DPV and References
DPV NO: 176 September 1977
Family: Secoviridae
Genus: Nepovirus
Species: Artichoke Italian latent virus | Acronym: AILV
Artichoke Italian latent virus
G. P. Martelli Istituto di Patologia vegetale, Università di Bari, 70126 Bari, Italy
G. L. Rana Istituto di Patologia vegetale, Università di Bari, 70126 Bari, Italy
V. Savino Istituto di Patologia vegetale, Università di Bari, 70126 Bari, Italy
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by Majorana & Rana (1970) and Vovlas, Martelli
& Quacquarelli (1971).
- An RNA-containing virus with isometric particles c. 30 nm in diameter, which sediment as three components when centrifuged. It is transmitted in soils by the nematode Longidorus apulus and readily by inoculation of sap to a wide range of hosts. In nature it infects both herbaceous and woody plants. Found in southern Italy and Bulgaria.
Main Diseases
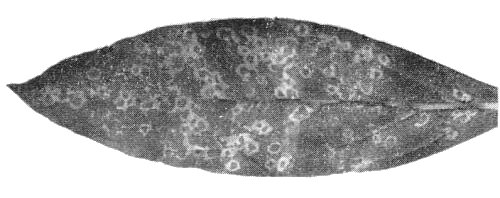
Latent in artichoke (Cynara scolymus) although occasionally isolated from stunted plants with mild yellowing of the leaves (Martelli & Rana, 1975). Causes chlorotic mottle often accompanied by bright yellow spots in the leaves of chicory (Cichorium intybus) (Vovlas et al., 1971; Vovlas & Roca, 1975) (Fig. 1), severe leaf malformations and stunting in pelargonium (Pelargonium zonale) (Vovlas, 1974) (Fig. 2), and fanleaf-like symptoms and reduced growth in grapevine (Jankulova et al., 1976).
Geographical Distribution
Reported from southern Italy and Bulgaria.
Host Range and Symptomatology
Besides the species mentioned above, the virus has been isolated from naturally infected gladiolus and sowthistle (Sonchus arvensis) plants showing yellow rings and line pattern on the leaves (Savino et al., 1977) and from a number of symptomless weeds (Crepis neglecta, Helminthia echioides, Hypochaeris aetnensis, Lactuca virosa, Urospermum dalechampii, Lamium amplexicaule, Sonchus spp.) growing in infected artichoke fields (Quacquarelli, Rana & Martelli, 1976). Transmitted experimentally by inoculation of sap to 63 species in 12 dicotyledonous families (Majorana & Rana, 1970; Vovlas et al., 1971; Vovlas, 1974; Vovlas & Roca, 1975; Savino et al., 1977).
- Diagnostic species
- Phaseolus vulgaris (French bean), cv. La Victoire.
Chlorotic and necrotic lesions develop in inoculated leaves.
Systemic symptoms consist of necrosis of the growing tip or
severe chlorotic/necrotic mottle of trifoliolate leaves.
Systemic necrosis is followed by recovery.
- Gomphrena globosa (globe amaranth). Necrotic, whitish ring-shaped local lesions (Fig. 3) occasionally followed by systemic necrosis and deformation of the leaves.
- Cucumis sativus (cucumber). Chlorotic and/or necrotic local lesions followed by severe systemic mosaic, deformation of the leaves and necrotic spots. Some isolates induce formation of enations on the lower leaf surface 2-3 weeks after inoculation.
- Nicotiana tabacum (tobacco) type White Burley. Chlorotic spots with necrotic ring-like patterns in the inoculated leaves. Systemic symptoms consist of necrotic and/or chlorotic ringspot and line patterns (Fig. 5).
- Gomphrena globosa (globe amaranth). Necrotic, whitish ring-shaped local lesions (Fig. 3) occasionally followed by systemic necrosis and deformation of the leaves.
- Propagation species
- Cucurbita pepo, Chenopodium quinoa and several
varieties of Phaseolus vulgaris are good sources of
virus for purification. C. pepo is suitable for
maintaining virus cultures.
- Assay species
- Phaseolus vulgaris cv. La Victoire is a satisfactory local lesion host. With some isolates Gomphrena globosa also is useful.
Strains
Although isolates differ in the symptoms induced in herbaceous hosts, these differences are not consistent enough to be used for identification of specific strains (Savino et al., 1977).
Transmission by Vectors
Transmitted efficiently by a Dorylaimoid nematode formerly identified as Longidorus attenuatus (Rana & Roca, 1975; Vovlas & Roca, 1975; Roca et al., 1975) but now recognized as a new species, Longidorus apulus (Lamberti & Bleve Zacheo, 1977). In transmission experiments in which viruliferous hand-picked nematodes were transferred from chicory to chicory (Vovlas & Roca, 1975) and from artichoke to tomato (Roca et al., 1975), about 60% and up to 67% of bait seedlings respectively, were infected.
Transmission through Seed
Not detected in chicory (Vovlas et al., 1971)
Transmission by Dodder
No information.
Serology
The virus is a good immunogen yielding antisera with titres up to 1/1024. In gel-diffusion tests a single precipitin band is formed and in immunoelectrophoresis in agar in veronal buffer pH 8.6, ionic strength 0.1, only one component migrating to the anode is resolved (Savino et al., 1977).
Relationships
All isolates studied were serologically indistinguishable (Savino et al., 1977). No relationship was detected to 28 viruses with isometric particles including the following members of the nepovirus group: grapevine fanleaf, arabis mosaic, tomato black ring (English and Scottish strains), tobacco ringspot, tomato ringspot, peach rosette mosaic, raspberry ringspot (English and Scottish strains), strawberry latent ringspot, grapevine chrome mosaic, cocoa necrosis, cherry leafroll, grapevine Bulgarian latent, myrobalan latent ringspot (Vovlas et al., 1971; Savino et al., 1977).
Stability in Sap
Minor differences were reported in determinations made at different times for different isolates. In expressed sap of C. quinoa, infectivity is lost after dilution to 10-2-10-5, heating for 10 min at 55-60°C or storing for 3-14 days at 4°C (Vovlas et al., 1971; Vovlas, 1974; Jankulova et al., 1976; Savino et al., 1977).
Purification
Not all isolates are readily purified (Vovlas, 1974). Satisfactory purification of stable isolates is achieved by clarification of expressed sap with magnesium-activated bentonite (Dunn & Hitchborn, 1965) used in different amounts according to the source host (i.e. 8% with C. pepo, 10% with C. quinoa and 12% with P. vulgaris), followed by cycles of differential centrifugation and sucrose density gradient centrifugation (Jankulova et al., 1976; Savino et al., 1977). Average yield of virus is c. 10 A1cm,260 units/100 g of infected material.
Properties of Particles
In sucrose density gradient and analytical ultracentrifugation purified virus preparations separate into three classes of particle: empty protein shells without RNA (T) sedimenting at about 55 S, and two nucleoproteins (M and B) containing different amounts of RNA and sedimenting at 96 S (M) and 121 S (B) (Fig. 4).
A260/A280: 0.75 (T), 1.53 (M), 1.7 (B).
The virus is unstable in CsCl (Quacquarelli et al., 1976a; Jankulova et al., 1976; Savino et al., 1977). Virus particles release infective RNA when frozen at -25°C and thawed, or when heated at 61°C under the conditions described by Quacquarelli, Piazzolla & Vovlas (1972) and Quacquarelli et al. (1976b).
Particle Structure
Particles are isometric, c. 30 nm in diameter with angular outlines. T particles are penetrated by negative stain. Partial penetration by neutral sodium phosphotungstate can be detected in preparations of M and B particles.
Particle Composition
Nucleic acid: Single-stranded RNA comprising 34% (M) and 41% (B) of the particle weight, calculated according to Reichmann’s (1965) formula. Molar percentages of nucleotides in RNA from unfractionated virus (artichoke isolate) are about: G24: A26: C19: U31 (Savino et al., 1977). Electrophoresis in 2.4% polyacrylamide gel separates RNA from unfractionated virus into two components of M. Wt 2.4 x 106 (RNA-1) and 1.5 x 106 (RNA-2). RNA-1 is present in B particles and RNA-2 in M particles. Neither species of RNA is infective alone, but infectivity is restored by mixing RNA-1 and RNA-2 (Jankulova et al., 1976; Quacquarelli et al., 1976a; Savino et al., 1977). Infective RNA is easily obtained from purified virus preparations in 0.02 M (Na-K) phosphate buffer containing 0.1 M NaCl by freezing at -25°C and thawing, or by heating at 61°C for 90 sec (Jankulova et al., 1976; Quacquarelli et al., 1976a; Savino et al., 1977). The single-phase phenol/SDS method (Diener & Schneider, 1968) is also useful.
Protein: In 7% polyacrylamide gels containing 0.1% SDS the protein migrates as one component of M. Wt 54,000 (Jankulova et al., 1976; Savino et al., 1977).
Relations with Cells and Tissues
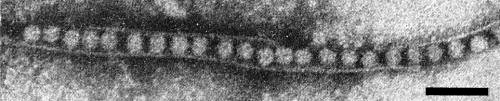
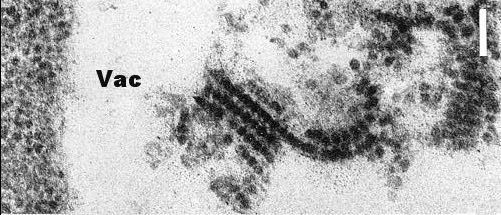
In systemically infected cucumber, rows of virus particles enclosed within membraneous tubules were found both in negatively stained mounts by the ‘squash homogenate’ technique (Walkey & Webb, 1968) (Fig. 6) and in thin sectioned parenchyma cells (Vovlas et al., 1971) (Fig. 7). Similar structures were also observed at the base of the odontostyle of nematode vectors that had fed on infected C. quinoa, thus suggesting that particle-containing tubules occur in the roots of this host (Taylor, Robertson & Roca, 1976). Particles contained within tubules do not adsorb at any point of the nematode's alimentary tract and probably pass into the intestine, whereas unprotected particles are specifically adsorbed onto the inner surface of the odontostyle of the vector (Taylor et al., 1976).
Notes
The virus has biological, ecological, epidemiological and physico-chemical characteristics typical of the nepoviruses. Its major distinctive characters are its lack of serological relatedness to any of the other known members of the group and its transmission by a Longidorus species not hitherto known as a virus vector. Based on its physico-chemical characters and hydrodynamic behaviour, artichoke Italian latent virus has been assigned to a subgroup containing tomato black ring and related viruses (Martelli, 1975; Quacquarelli et al., 1976a).
Figures

Naturally infected pelargonium plant: growth is stunted, leaves are small, severely malformed and have abnormally long petioles.

Schlieren diagram of a partially purified virus preparation after 15 min at 30,000 rev/min: three virus-specific components (T, M and B) and some normal plant material (H) are visible. Sedimentation is from left to right.
References list for DPV: Artichoke Italian latent virus (176)
- Diener & Schneider, Archs Biochem. Biophys. 124: 401, 1968.
- Dunn & Hitchborn, Virology 25: 171, 1965.
- Jankulova, Savino, Gallitelli, Quacquarelli & Martelli, Abstr. Proc. 6th Conf Internatl. Counc. Grapevine Viruses Cordova 1976: 24, 1976.
- Lamberti & Bleve Zacheo, Nematol. Mediterranea 5: 73, 1977.
- Majorana & Rana, Phytopath. Mediterranea 9: 193, 1970.
- Martelli, In Nematode Vectors of Plant Viruses p.223, ed. F. Lamberti, C. E.Taylor & J. W. Seinhorst, London & New York: Plenum, 1975.
- Martelli & Rana, Atti. 2° Congr. Internaz. Carciofo, Bari 1973: 811, 1975.
- Quacquarelli, Rana & Martelli, Poljopr. znanst. Smotra 39: 561, 1976.
- Quacquarelli, Piazzolla & Vovlas, J. gen. Virol. 17: 147, 1972.
- Quacquarelli, Gallitelli, Savino, Piazzolla & Martelli, Abstr. Proc. 6th Conf. Internatl. Counc. Grapevine Viruses Cordova 1976: 10, 1976a.
- Quacquarelli, Gallitelli, Savino & Martelli, J. gen. Virol. 32: 349, 1976b.
- Rana & Roca, Atti. 2° Congr. Internaz. Carciofo Bari 1973: 855, 1975.
- Reichmann, Virology 25: 166, 1965.
- Roca, Martelli, Lamberti & Rana, Nematol. Mediterranea 3: 91, 1975.
- Savino, Gallitelli, Jankulova & Rana, Phytopath. Mediterranea 16: 41, 1977.
- Taylor, Robertson & Roca, Nematol. Mediterranea 4: 23, 1976.
- Vovlas, Phytopath. Mediterranea 13: 139, 1974.
- Vovlas & Roca, Nematol. Mediterranea 3: 83, 1975.
- Vovlas, Martelli & Quacquarelli, Phytopath. Mediterranea 10: 244, 1971.
- Walkey & Webb, J. gen. Virol. 3: 311, 1968.