Details of DPV and References
DPV NO: 177 September 1977
Family: Tombusviridae
Genus: Panicovirus
Species: Panicum mosaic virus | Acronym: PMV
Panicum mosaic virus
C. L. Niblett Department of Plant Pathology, Kansas State University, Manhattan, Kansas 66506, USA
A. Q. Paulsen Department of Plant Pathology, Kansas State University, Manhattan, Kansas 66506, USA
R. W. Toler Department of Plant Sciences, Texas A&M University, College Station, Texas 77843, USA
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by
Sill & Pickett (1957),
Lee (1973), and
Niblett & Paulsen (1975).
Synonym
- St. Augustine decline virus
-
An RNA-containing virus with isometric particles 25-30 nm in diameter. It is usually associated with a serologically distinct satellite-like virus which is 15-18 nm in diameter. Panicum mosaic virus is readily sap-transmissible and infects only species in the Gramineae. No vector has been identified. The virus occurs in the USA and Mexico.
Main Diseases
The type strain (from Kansas) causes mild mosaic symptoms in many grasses but has not been found occurring naturally in economic crop species. The St. Augustine decline (SAD) strain causes significant economic losses in St. Augustinegrass (Stenotaphrum secundatum) lawns (McCoy et al., 1969).
Geographical Distribution
The virus has been reported in the USA and Mexico. The type strain occurs in Kansas, and the SAD isolates occur in Texas, Louisiana, and Mexico (Holcomb et al., 1972).
Host Range and Symptomatology
The host range of both strains is restricted to the Gramineae and includes many symptomless hosts. Numerous dicotyledonous species have been inoculated, but none were susceptible (McCoy et al., 1969, Niblett & Paulsen, 1975).
-
Diagnostic species
- Zea mays
(maize). Several inbred corn lines are susceptible to the type strain. A mild mosaic develops 7-10 days after inoculation (Fig. 1). Ohio 28 and N28 are useful indicator and purification hosts. The SAD strain does not infect maize. - Stenotaphrum secundatum (St. Augustinegrass). Infected only by the SAD isolates. A chlorotic mottling occurs 14-21 days after inoculation (Fig. 2). General chlorosis, internodal shortening, and finally, leaf and stolon necrosis occur in infected lawns.
-
Propagation species
- Zea mays
(especially N28) is a useful propagation host for the type strain, Stenotaphrum secundatum for the SAD isolates.Assay species
- The SAD isolates cause local lesions on Panicum dichtomiflorum (Abu-Samah & Holcomb, 1975; 1976). This has not been tested with the type strain. Both strains may be assayed on Panicum miliaceum and Setaria italica, by determining the proportion of plants becoming systemically infected.
Strains
The type and SAD strains may be differentiated by the above host reactions. Isolates of the SAD strain may be further distinguished serologically (Holcomb, 1974) and by host reactions (G. E. Holcomb, personal communication).
Transmission by Vectors
None found. The apple grain aphid (Rhopalosiphum prunifoliae) is not a vector of the type strain (Sill & Pickett, 1957). Soil transmission experiments with an SAD isolate were negative (McCoy et al., 1969).
Transmission through Seed
None found for the type strain in Panicum virgatum, P. maximum, Digitaria sanguinalis, Setaria italica, S. lutescens, or Briza maxima (Sill & Desai, 1960, Niblett & Paulsen, 1975). However, seed transmission was reported for an SAD isolate in Setaria italica, cv. Red Siberian (Wilson, 1974).
Transmission by Dodder
Not tested.
Serology
Rabbits injected with purified preparations of the 109 S particles yield antisera producing a single precipitin band with titres up to 1/1024 in agar double diffusion tests. Antisera prepared against preparations containing both 109 S and 42 S particles produce an additional band closer to the antiserum well when allowed to react with crude sap from infected plants or with unfractionated purified virus. Antisera prepared to the separated particles indicate that they are unrelated serologically (C. L. Niblett, unpublished data).
Relationships
The 109 S particles of the type strain and the SAD strain are closely serologically related but distinguishable (Holcomb, 1974; C. L. Niblett, unpublished data). Relationships to other grass viruses have recently been suggested. Purified and crude preparations of the type strain react with antiserum to phleum mottle virus but not with antiserum to cocksfoot mild mosaic virus (C. L. Niblett, unpublished data - antisera kindly provided by Drs W. Huth and H. L. Paul). Also, there is a serological relationship between the type strain and molinia streak virus (W. Huth, H. L. Paul & G. Querfurth, unpublished data).
Stability in Sap
In crude leaf extracts of the type strain the thermal inactivation point (10 min) is 85°C; the dilution end-point is 10-5; and infectivity is retained in refrigerated, desiccated tissue for at least 12 years (Canares, 1967; Niblett & Paulsen, 1975). Purified virus seems to be less stable (Niblett & Paulsen, 1975). A thermal inactivation point of 60°C and a dilution end-point of 10-4 were reported for an SAD isolate (Lee, 1973).
Purification
The virus is readily purified from maize (type strain) or St. Augustinegrass (SAD strain) by Steere’s chloroform-butanol procedure and/or precipitation by polyethylene glycol M. Wt 6000 followed by differential and/or density gradient centrifugation. This yields A260 = 0.5/g tissue for the type strain (Niblett & Paulsen, 1975).
Properties of Particles
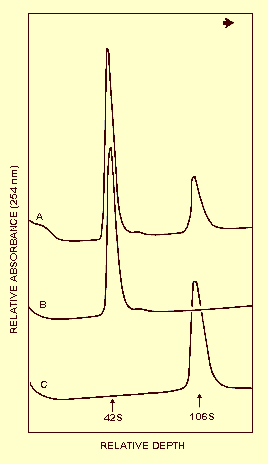
Purified preparations of the type strain and of one isolate of the SAD strain contain two classes of nucleoprotein particles. Sedimentation coefficients of 42 S and 109 S were determined for the type strain by density gradient centrifugation (Fig. 3). Only the 109 S particles are infective (Niblett & Paulsen, 1975; Buzen & Niblett, 1976). A sedimentation coefficient of 102 S was reported for an SAD isolate (Lee, 1973).
A260/A280: 1.66 for the 109 S particles and 1.72 for the 42 S particles of the type strain (Niblett & Paulsen, 1975; C. L. Niblett, unpublished data); 1.56 has been recorded for an SAD strain (C. L. Niblett, unpublished data).
Particle Structure
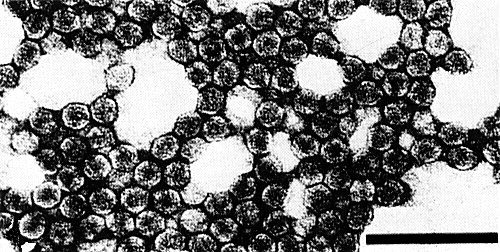
The 109 S particles are isometric, about 25-30 nm in diameter (Fig. 4); the 42 S particles are isometric, about 15-18 nm in diameter (Fig. 5).
Particle Composition
Nucleic acid: the 109 S particles contain a 28 S RNA, which is infective. The 42 S particles contain two RNA species which sediment at 14 S and 34 S and are not infective (Buzen & Niblett, 1976). Base compositions have not been determined.
Protein: the protein subunit of the 109 S particles has a M. Wt of about 29,000 and that of the 42 S particles is about 15,500 (C. L. Niblett, unpublished data).
Relations with Cells and Tissues
This has been examined for an SAD isolate in resistant (Floratam) and susceptible St. Augustinegrass and in Setaria italica (Wilson, 1974). Virus particles occurred in crystals, and inclusion bodies were observed in infected plants. Neither occurred with regularity in Floratam. Cytopathic effects such as disruption of the mitochondria and of the tonoplast were apparent in infected St. Augustinegrass.
Notes
The role and origin of the 42 S particles are unclear but their lack of infectivity and lack of serological relationship to the 109 S particles suggest that they may represent a satellite virus analogous to that associated with tobacco necrosis virus (Kassanis, 1970).
There are several grass viruses with similar morphology and host range and their relationships and affinities are under investigation. In addition to those mentioned under Relationships, a virus of maize reported by Lapierre & Signoret (1975) is of interest because of its association with two kinds of particles 25-30 nm and 14-18 nm in diameter.
Figures
References list for DPV: Panicum mosaic virus (177)
- Abu-Samah & Holcomb, Pl. Dis. Reptr 59: 999, 1975.
- Abu-Samah & Holcomb, Phytopathology 66: 215, 1976.
- Buzen & Niblett, Proc. Am. Phytopath. Soc. 3: 250, 1976.
- Canares, M.S. Thesis, Kansas State University, 1967.
- Holcomb, Proc. Am. Phytopath. Soc. 1: 21, 1974.
- Holcomb, Derrick, Carver & Toler, Pl. Dis. Reptr 56: 69, 1972.
- Kassanis, CMI/AAB Descriptions of Plant Viruses 15, 4 pp., 1970.
- Lapierre & Signoret, Annales de Phytopathologie 7: 232, 1975.
- Lee, Ph.D. Dissertation, Texas A&M Univ., 1973.
- McCoy, Toler & Amador, Pl. Dis. Reptr 53: 955, 1969.
- Niblett & Paulsen, Phytopathology 65: 1157, 1975.
- Sill & Desai, Pl. Dis. Reptr 44: 487, 1960.
- Sill & Pickett, Pl. Dis. Reptr 41: 241, 1957.
- Wilson, Ph.D. Dissertation, Texas A&M Univ., 1974.