Details of DPV and References
DPV NO: 18 June 1970
Family: Secoviridae
Genus: Nepovirus
Species: Tomato ringspot virus | Acronym: ToRSV
There is a more recent description of this virus: DPV 290
Tomato ringspot virus
R. Stace-Smith Canada Agriculture Research Station, Vancouver, B.C., Canada
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by
Price (1936).
- Selected synonyms
- Tobacco ringspot virus No. 2 (Rev. appl. Mycol. 15: 831)
- Nicotiana virus 13 (Rev. appl. Mycol. 36: 303)
- Annulus zonatus (Rev. appl. Mycol. 28: 514)
- Peach yellow bud mosaic virus (Rev. appl. Mycol. 24: 65)
- Grape yellow vein virus (Rev. appl. Mycol. 35: 286)
- Nicotiana virus 13 (Rev. appl. Mycol. 36: 303)
- An RNA-containing virus with isometric particles about 28 nm in diameter. It is readily transmissible by inoculation of sap and has a wide host range, including both woody and herbaceous plants. It is transmitted by the nematode Xiphinema americanum.
Main Diseases
Causes mosaic or ringspot symptoms in tobacco, Pelargonium, Hydrangea, raspberry and blackberry; rasp leaf in cherry; yellow bud mosaic in peach; yellow vein in grapevine; stunt or stub head in Gladiolus; and decline of raspberry.
Geographical Distribution
Field spread restricted to the temperate regions of North America where the nematode vector Xiphinema americanum is prevalent. Endemic in native wild hosts along the California coast (Frazier, Yarwood & Gold, 1961). Has probably been distributed in ornamental crops to other parts of the world although to date there have been no confirmed reports.
Host Range and Symptomatology
Experimental host range is wide; spp. in more than 35 dicotyledonous and monocotyledonous families are susceptible. In nature, the virus occurs mostly in ornamentals and woody or semiwoody plants. Transmissible by sap inoculation, readily to herbaceous hosts but with difficulty to woody hosts.
Diagnostic species
- Chenopodium amaranticolor
(Fig. 1)
and C. quinoa. Small
chlorotic local lesions; systemic apical necrosis.
- Cucumis sativus (cucumber). Local chlorotic spots; systemic chlorosis and mottle.
- Phaseolus vulgaris (bean). Chlorotic local lesions; systemic rugosity and necrosis of tip leaves (Fig. 2).
- Nicotiana tabacum (tobacco). Necrotic local spots or rings (Fig. 4); systemic etched ring and line patterns. Leaves produced later are symptomless but contain virus.
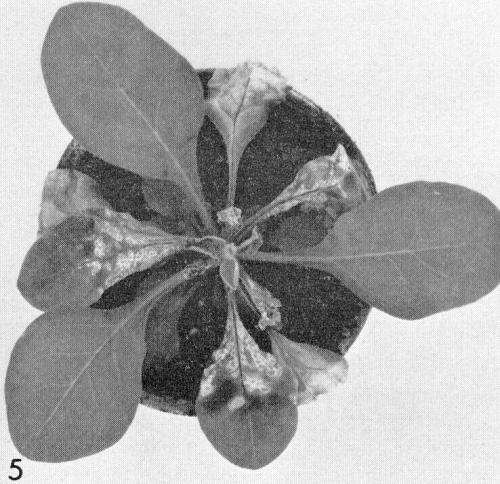
- Petunia hybrida (Fig. 5). Local necrotic lesions; necrotic collapse of young leaves.
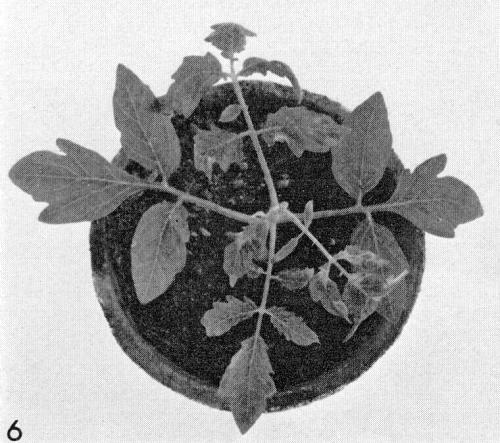
- Lycopersicon esculentum (tomato). Local necrotic flecks; systemic mottle and necrosis (Fig. 6).
- Vigna sinensis (cowpea; Fig. 7). Chlorotic or necrotic local lesions; most isolates cause systemic tip necrosis.
- Cucumis sativus (cucumber). Local chlorotic spots; systemic chlorosis and mottle.
- Propagation species
- Nicotiana tabacum or woody plants such as raspberry or currant are
suitable for maintaining cultures; Cucumis sativus (cucumber) or Petunia
are good sources of virus for purification.
- Assay species
- Vigna sinensis, Nicotiana tabacum, Chenopodium amaranticolor, and C. quinoa are useful local lesion hosts. Cucumber is useful as a source and bait plant for nematode transmission experiments (Téliz, Grogan & Lownsbery, 1966).
Strains
Variants giving slightly different symptoms in herbaceous hosts have been
found. The best known variants, distinguished not by host reactions but on the
basis of the field disease attributed to the virus, are as follows:
Tobacco strain = tobacco ringspot virus No. 2 of
Price (1936).
Occurred spontaneously in tobacco seedlings in a greenhouse. Source Eastern
USA.
Peach yellow bud mosaic strain. Occurs naturally in almond, apricot
and peach. Herbaceous host reactions similar to those caused by Price’s strain
(Karle, 1960)
from which it cannot be distinguished serologically
(Cadman & Lister, 1961).
Source Western USA.
Grape yellow vein strain. Occurs naturally in grape. Herbaceous host reactions similar to above strains but differs in causing no top necrosis in cowpea; also differs somewhat serologically. Source Western USA.
Transmission by Vectors
Transmitted by adults and three larval stages of the nematode Xiphinema americanum. Nematodes can acquire virus within 1 h and can inoculate it into healthy plants within 1 h. Single nematodes can transmit (Téliz et al., 1966). Tobacco ringspot and tomato ringspot viruses can both be transmitted by the same single nematode (Fulton, 1967).
Transmission through Seed
Transmitted occasionally through tobacco and raspberry seed, frequently through soybean (Kahn, 1956) and strawberry (Mellor & Stace-Smith, 1963) seed. Pollen transmission not investigated.
Transmission by Dodder
A virus thought to be tomato ringspot virus was not transmitted by Cuscuta californica, C. subinclusa, or C. campestris (Bennett, 1944).
Serology
The virus is moderately immunogenic; antiserum titres in excess of 1:1000 may be attained. Diagnostic serological reactions can be obtained in ring precipitin, micro precipitin, tube precipitin, gel-diffusion or complement fixation tests. Antisera prepared by intravenous or intramuscular injections give a single band of precipitate in gel-diffusion tests.
Relationships
Tomato ringspot virus is not related serologically to the nematode-borne
viruses that occur in Europe
(Cadman & Lister, 1961),
nor to tobacco ringspot
(Tall, Price & Wertman, 1949)
or tomato top necrosis
(Bancroft, 1968),
two similar viruses that occur in North America. Prior to the widespread
use of serology, plant protection tests were used to distinguish tomato ringspot
and tobacco ringspot viruses; reciprocal tests in recovered tobacco plants
(Price, 1936)
or petunia plants
(Cadman & Lister, 1961)
show lack of
relationship.
Unilateral protection occurs between tomato ringspot and
elm mosaic viruses
but serological comparisons, reveal no cross reactions and vector relationships
suggest that the two viruses are distinct
(Fulton & Fulton, 1970).
The tobacco and peach yellow bud mosaic strains do not differ antigenically but the grape yellow vein strain, although closely related to the others, may be distinguished by the formation of spurs in gel-diffusion tests (Gooding, 1963; Téliz et al., 1966).
Stability in Sap
In tobacco sap, the virus loses infectivity after 10 min at about 58%, 2 days at 20°C, 3 weeks at 4°C, or several months at -20°C. Dilution end-point of sap from inoculated tobacco leaves or cucumber cotyledons is 10-3; that of sap from systemically infected tobacco is less than 10-1 (Stace-Smith, 1962).
Purification
The virus is relatively unstable and is destroyed by many routine
purification procedures. Two methods seem satisfactory:
1. Gooding (1963).
Freeze leaves and stems from infected bean plants and
homogenize each 100 g in 50 ml distilled water containing 0.75 g sodium
ascorbate. Squeeze through cheesecloth, add n-butanol to 8.5% (v/v) and
centrifuge at low speed. Concentrate by four cycles of high- and low-speed
centrifugation, resuspending the pellets obtained at high speed in 0.02 M
phosphate buffer, pH 7.2. Subject the final product to sucrose density gradient
centrifugation.
2. Stace-Smith (1966). Extract each 100 g infected cucumber tissue in 150 ml 0.5 M borate buffer, pH 6.7. Squeeze extract through cheesecloth, freeze overnight, thaw, and centrifuge at low speed. Add granular ammonium sulphate, 15 g/100 ml extract, stir overnight and centrifuge at low speed. Concentrate the supernatant fluid by two cycles of high- and low-speed centrifugation, resuspending the pellets obtained at high speed in 0.01 M versene buffer, pH 7.0. Subject the final product to sucrose density gradient centrifugation. Yield is 10-20 mg/kg of infected leaves.
Properties of Particles
Purified preparations contain two major classes of particles, empty protein
shells without RNA (T) and the infective nucleoprotein (B). The two classes of
particle are serologically indistinguishable; only the B particles are infective
(Stace-Smith, 1966).
Sedimentation coefficients (s20,w) at infinite dilution (svedbergs):
53 (T), 126-128 (B).
Molecular weights (daltons): About 3.2 x 106 (T), 5.5 x 106 (B).
Isoelectric point: c. pH 5.1.
Electrophoretic mobility: -2.60 x 10-5 cm2 volt-1 sec-1
in 0.1 ionic strength phosphate buffer, pH 7.38; or
1.59 x 10-5 cm2 volt-1 sec-1 at pH 6.55 (B and T).
A260/A280: about 1.05 (T), 1.8 (B).
Absorbance at 260 nm (1 mg/ml, 1 cm light path): about 10.0 (B).
Particle Structure
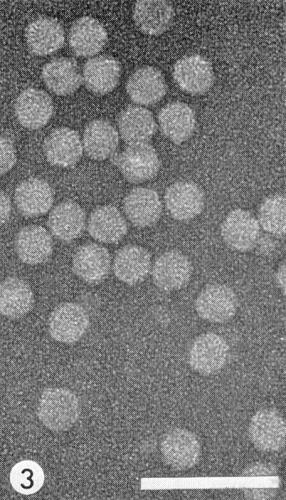
Particles are isometric, about 28 nm in diameter (Fig. 3). They disintegrate somewhat when mounted in phosphotungstate for electron microscopy. They are penetrated by phosphotungstate and contrast poorly.
Particle Composition
RNA: Only the 126 S particle contains RNA. Molecular weight about
2.3 x 106. Molar percentages of nucleotides: G 26; A 23; C 22; U 29.
RNA is about 40% of the particle weight
(Tremaine & Stace-Smith, 1968).
Protein: About 60% of particle weight. For amino acid composition, see Tremaine & Stace-Smith (1968).
Relations with Cells and Tissues
Particles thought to be those of tomato ringspot virus were observed in low concentration in the cytoplasm and plasmodesmata of infected leaf tissue of Datura stramonium (de Zoeten & Gaard, 1969).
Notes
Tomato ringspot virus is similar in size, shape, physical properties, host
reaction and patchy distribution in the field to other
nepoviruses
but is
serologically unrelated to any of them. Serological tests are therefore
essential for positive identification of isolates thought to belong to this
group of viruses. The geographical distribution, natural host range, and vector
relationships of tomato ringspot virus closely parallel those of
tobacco ringspot virus.
If antisera are not available to distinguish isolates of these
viruses, differential host reactions may be useful
(McLean, 1962).
The name tomato ringspot has been applied to two apparently unrelated viruses, one isolated from tobacco seedlings by Price (1936) and the other from tomato plants by Samson & Imle (1942). Price’s isolate, or isolates serologically related to it, has been used for physical, chemical, and biological characterization of tomato ringspot virus. The isolate of Samson & Imle was probably related not to Price’s tomato ringspot virus but to tomato top necrosis virus (Bancroft, 1968).
Figures
References list for DPV: Tomato ringspot virus (18)
- Bancroft, Phytopathology 58: 1360, 1968.
- Bennett, Phytopathology 44: 905, 1944.
- Cadman & Lister, Phytopathology 51: 29, 1961.
- de Zoeten & Gaard, J. Cell Biol. 40: 814, 1969.
- Frazier, Yarwood & Gold, Pl. Dis. Reptr 45: 649, 1961.
- Fulton, Phytopathology 57: 535, 1967.
- Fulton & Fulton, Phytopathology 60: 114, 1970.
- Gooding, Phytopathology 53: 475, 1963.
- Kahn, Phytopathology 46: 295, 1956.
- Karle, Phytopathology 50: 466, 1960.
- McLean, Pl. Dis. Reptr 46: 877, 1962.
- Mellor & Stace-Smith, Can. J. Bot 41: 865, 1963.
- Price, Phytopathology 26: 665, 1936.
- Samson & Imle, Phytopathology 32: 1037, 1942.
- Stace-Smith, Can. J. Bot. 40: 905, 1962.
- Stace-Smith, Virology 29: 240, 1966.
- Tall, Price & Wertman, Phytopathology 39: 288, 1949.
- Téliz, Grogan & Lownsbery, Phytopathology 56: 658, 1966.
- Tremaine & Stace-Smith, Virology 35: 102, 1968.