Details of DPV and References
DPV NO: 180 September 1977
Family: Bromoviridae
Genus: Bromovirus
Species: Brome mosaic virus | Acronym: BMV
There is a more recent description of this virus: DPV 405
This is a revised version of DPV 3
Brome mosaic virus
L. C. Lane Dept. of Plant Pathology, University of Nebraska, Lincoln, NE 68583, USA
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by McKinney, Fellows & Johnston (1942).
Selected synonyms
- Weidelgrasmosaik-Virus (Rev. appl. Mycol. 39: 589)
- Ryegrass streak virus (Rev. appl. Mycol. 44: 152)
- Trespenmosaik-Virus (Rev. appl. Mycol. 44: 2517)
- Marmor graminis (Rev. appl. Mycol. 23: 427)
- Ryegrass streak virus (Rev. appl. Mycol. 44: 152)
-
An RNA-containing virus about 26 nm in diameter. It is easily transmitted mechanically and infects many species of Gramineae and several dicotyledonous plants. It is of particular interest for its ease of purification, for its divided genome, and because it is readily reassembled from RNA and protein.
Main Diseases
Causes mosaic of bromegrass (Bromus inermis) and other wild grasses. The virus has not caused documented economic losses.
Geographical Distribution
Central USA, Eastern Europe, South Africa.
Host Range and Symptomatology
Brome mosaic virus infects species in roughly 50 genera of the Gramineae. Among dicotyledonous plants its host range is restricted to a few genera in about six families. A complete host range has been tabulated (Lane, 1974).
-
Diagnostic species
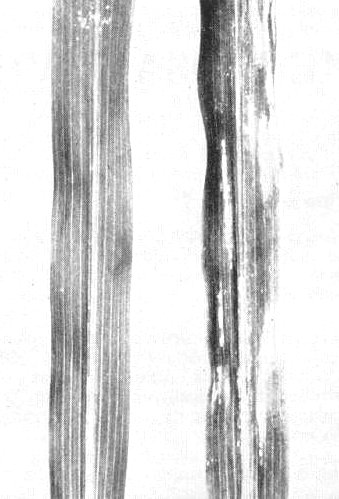
- Zea mays
(maize). Seedlings of most varieties show primary lesions or streaks (Fig. 1) followed by necrosis and death (Fig. 2). - Chenopodium spp. Brome mosaic is one of the few grass viruses that produce local lesions on
Chenopodium amaranticolor, C. hybridum
(Fig. 3) and C. quinoa.
Propagation species
- Hordeum vulgare
(barley). Mild mosaic. -
Assay species
- Chenopodium hybridum
(Rochow, 1959) (Fig. 3) is a local lesion host.
Strains
The isolate of McKinney et al. (1942) is regarded as the type strain. Clearly distinct natural isolates have not been found, although slight differences in stability and other properties have been noted. Natural mutants, such as electrophoretic variants, and chemically induced mutants can be selected (Lane, 1974).
Transmission by Vectors
Nematodes of the genus Xiphinema transmitted the virus in the laboratory (Schmidt, Fritsche & Lehmann, 1963; Fritsche, 1975) but vectors have not been demonstrated in the field. Attempts to transmit the virus with aphids and mites have been unsuccessful (Lane, 1974). Broad bean mottle and cowpea chlorotic mottle, the other two bromoviruses, are transmitted by beetles (Walters & Dodd, 1969; Walters & Surin, 1973).
Transmission through Seed
All reports have been negative (Lane, 1974).
Transmission by Dodder
None reported.
Serology
Antisera with dilution end-points greater than 1/1000 have been prepared but antibody titres drop sharply after reaching a maximum (Moorhead, 1956). Serological studies are complicated by the instability of the virus above pH 6 and its tendency to interact ionically with the sulphate groups of agar and agarose below this pH. Under appropriate conditions, antisera specific to intact virus particles or to the dissociated coat protein can be prepared (von Wechmar & van Regenmortel, 1968).
Relationships
Brome mosaic virus is a distant serological relative of cowpea chlorotic mottle virus (Scott & Slack, 1971). The ability of RNAs 1 and 2 from brome mosaic virus and RNA 3 from cowpea chlorotic mottle virus to form a viable genetic hybrid, albeit with a much reduced growth rate and host range (Bancroft, 1972) also indicates a relationship. Similar physical properties and coat protein amino acid compositions indicate a relationship between brome mosaic and broad bean mottle viruses.
Stability in Sap
The properties of the virus in sap vary. On the average, infectivity survives 10 min at 80°C or dilution of 104 or 105.
Purification
Easily purified with or without an ultracentrifuge. A simple method is to grind barley tissue (roughly 2 weeks after infection) with an equal weight of 0.5 M sodium acetate buffer adjusted to pH 4.5 with acetic acid and then emulsify with a small amount of chloroform. Centrifuge the suspension for 10 min at 5000 rev/min. Filter the supernatant fluid and precipitate the virus by adding polyethylene glycol M.Wt 6000 to 6 % (w/v). After 15 min of stirring on ice, collect the precipitate by centrifuging 15 min at 5000 rev/min. Resuspend the pellet in distilled water to about 1/10 the original volume and emulsify with a few ml of chloroform. Centrifuge the emulsion 10 min at 5000 rev/min and precipitate virus from the upper phase by adding polyethylene glycol to 6% (w/v) and mixing with 1/10 volume of 5 M NaCl. After 15 min of stirring on ice, collect the precipitate by centrifugation and resuspend in 50 mM sodium acetate, 1 mM magnesium acetate, pH 5. Virus may be further purified by differential centrifugation (90 min at 40,000 rev/min and 5 min at 20,000 rev/min). Purified virus may be stored frozen by adding 1/20 volume of ethylene glycol as a cryopreservative. Contaminating nucleases gradually degrade RNA within the virus. Quality of virus preparations is best judged by analyzing the integrity of the RNA by gel electrophoresis.
Properties of Particles
The virus is stable from pH 3 to 6. Above pH 7 it swells and is degraded by contaminating nucleases (Incardona & Kaesberg, 1964). Divalent cations (e.g. 1 mM Mg2+) stabilize the virus above pH 6 (Brakke, 1963).
Sedimentation coefficient (s20, w): (87.3-0.47 c) S at pH 3-6 and (78.7-0.64 c) S at pH 7 and above (Incardona & Kaesberg, 1964), where c is the virus concentration in mg/ml.
M.Wt: 4.6 x 106 (Bockstahler & Kaesberg, 1962).
The isoelectric point varies considerably with ionic strength; it is pH 6.8 by isoelectric focusing (Rice & Horst, 1972).
Partial specific volume: 0.71 cm3/g (estimated, Bockstahler & Kaesberg, 1962).
Electrophoretic mobilities as a function of pH are given by Bockstahler & Kaesberg (1962) and Johnson, Wagner & Bancroft (1973).
Extinction coefficient (E(0.1%, 1cm)) at 260 nm, uncorrected for light-scattering is 5.15 (Bockstahler & Kaesberg, 1962).
A260/A280 is 1.75. Amax/min is 1.53 (L. C. Lane, unpublished data).
The buoyant density in CsCl is roughly 1.35 g/cm3 (Bancroft, 1971).
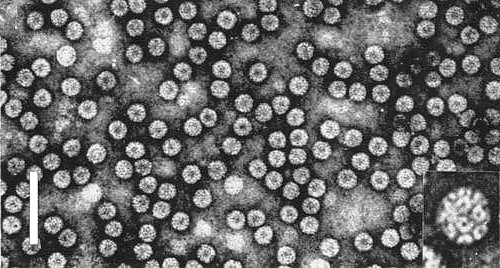
Particle Structure
Particles are isometric (Fig. 4) and roughly 26 nm in diameter with hollow centres roughly 8 nm in diameter (Anderegg, Wright & Kaesberg, 1963). The 180 protein subunits are clustered into hexamers and pentamers (Bancroft, Hills & Markham, 1967). The virus consists of three types of particle differing slightly in buoyant density, all of which are required for infectivity. Heavy particles contain RNA-1, light particles contain RNA-2 and those of intermediate density contain one molecule each of RNA-3 and RNA-4 (Lane & Kaesberg, 1971).
Particle Composition
Nucleic acid:
Single-stranded RNA, about 22% of the particle weight
(Bockstahler & Kaesberg, 1962).
G:A:C:U = 28:27:21:24
(Bockstahler & Kaesberg, 1965).
The RNA is readily isolated by phenol
extraction and consists of four RNA species of M.Wt 1.1 x 106 (RNA-1), 1.0 x 106
(RNA-2), 0.7 x 106 (RNA-3), and 0.3 x 106 (RNA-4), occurring in roughly, but not
exactly, equimolar ratio. RNA species 1, 2 and 3 are required to infect and RNA-3 contains the coat
protein gene
(Lane & Kaesberg, 1971).
RNA-4 is a nucleotide sequence derived from that of RNA-3
(Shih, Lane & Kaesberg, 1972).
RNA species 1 to 4 code respectively for proteins of M.Wt
1.2 x 105, 1.1 x 105, 0.35 x 105 and 0.2 x 105. The last
is the coat protein, which is preferentially translated from mixtures containing RNA-4
(Shih & Kaesberg, 1973,
1976).
All four RNA species can be charged at the 3' end with the amino acid tyrosine
by plant-derived amino acyl tRNA synthetases
(Hall, Shih & Kaesberg, 1972). All four 3' termini
have similar sequences
(Bastin et al., 1976).
The 3' terminal nucleotide sequence of RNA-4 (P. Kaesberg, personal communication) is:
161 A 160 C A C G C A G A C C U C U U A C A A G A 140 G U G U C U A G G U G C C U U U G A G A 120 G U U A C U C U U U G C U C U C U U C G 100 G A A G A A C C C U U A G G G G U U C G 80 U G C A U G G G C U U G C A U A G C A A 60 G U C U U A G A A U G C G U A C C G G G 40 U G U A C A G U U G A A A A A C A C U G 20 U A A A U C U C U A A A A G A G A C C A OHThe 5' terminal sequence of RNA-4 (Das Gupta et al., 1975) is:
Met Ser
Thr Ser
Gly Thr
Gly
7MeG p p p G U A
U U A A U A
A U G U C G A C U U C A G G A
A C U
G G U
10
20
30
Lys Met
Thr Arg Ala
Gln Arg Arg
A A G A U G A C U C G C
G C G C A G C G U C G
40
50
The 5' ends of all four RNA species are ‘capped’ with 7-methyl guanosine.
Protein: The coat protein can be isolated by disrupting the virus and precipitating the RNA with CaCl2. The protein weighs 20,300 daltons. Its amino acid composition is given by Stubbs & Kaesberg (1964). The N-terminal methionine in the amino acid sequence given in the previous section is replaced by an acetyl group in the ‘mature’ coat protein. The C-terminus is arginine (P. Kaesberg, personal communication).
Other components: The virus contains no polyamines (Nickerson & Lane, 1977).
Relations with Cells and Tissues
In tobacco protoplasts, the endoplasmic reticulum around the nucleus proliferates during the first 6 h of infection. Later, virus particles are found scattered throughout the cytoplasm. Some virus particles associate in a helical array around the outside of membranous tubules which are about 30 nm in diameter (Burgess, Motoyoshi & Fleming, 1974). In oat and barley leaves, photosynthetic tissue (mesophyll cells) is more severely affected than other tissues, and virus is often seen in chloroplast invaginations. Both hosts contain virus crystals at late stages in the disease. Chloroplasts degenerate in yellowed areas of the leaves (Paliwal, 1970).
A membrane-bound RNA polymerase, which is specific to infected tissue, may be isolated. A virus-induced protein of M. Wt 35,000 appears to be a component of the polymerase (Hariharasubramanian et al., 1973). The polymerase can be dissociated from the membrane with nonionic detergents and is stimulated by, but not dependent on, added brome mosaic virus RNA (Kummert & Semal, 1977).
Notes
Brome mosaic virus is distinguishable from most other viruses of Gramineae by its symptoms in maize and by its ability to infect several non-graminaceous hosts. In view of the ease of purification (it can be purified in high yield from naturally infected bromegrass), cursory physical characterization is advisable for purposes of identification.
Figures
References list for DPV: Brome mosaic virus (180)
- Anderegg, Wright & Kaesberg, Biophys. J. 3: 175, 1963.
- Bancroft, Virology 45: 830, 1971.
- Bancroft, J. gen. Virol. 14: 223, 1972.
- Bancroft, Hills & Markham, Virology 31: 354, 1967.
- Bastin, Dasgupta, Hall & Kaesberg, J. molec. Biol. 103: 737, 1976.
- Bockstahler & Kaesberg, Biophys. J. 2: 1, 1962.
- Bockstahler & Kaesberg, J. molec. Biol. 13: 127, 1965.
- Brakke, Virology 19: 367, 1963.
- Burgess, Motoyoshi & Fleming, Planta 117: 133, 1974.
- Dasgupta, Shih, Saris & Kaesberg, Nature, Lond. 256: 624, 1975.
- Fritsche, Archiv für Phytopathologie und Pflanzenschutz 11: 197, 1975.
- Hall, Shih & Kaesberg, Biochem. J. 129: 969, 1972.
- Hariharasubramanian, Hadidi, Singer & Fraenkel-Conrat, Virology 54: 190, 1973.
- Incardona & Kaesberg, Biophys. J. 4: 11, 1964.
- Johnson, Wagner & Bancroft, J. gen. Virol. 19: 263, 1973.
- Kummert & Semal, Virology 77: 212, 1977.
- Lane, Adv. Virus Res. 19: 151, 1974.
- Lane & Kaesberg, Nature New Biol. 232: 40, 1971.
- McKinney, Fellows & Johnston, Phytopathology 32: 331, 1942.
- Moorhead, Phytopathology 46: 498, 1956.
- Nickerson & Lane, Virology 81: 455, 1977.
- Paliwal, J. Ultrastruct. Res. 30: 491, 1970.
- Rice & Horst, Virology 49: 602, 1972.
- Rochow, Phytopathology 49: 126, 1959.
- Schmidt, Fritsche & Lehmann, Naturwissenschaften 50: 386, 1963.
- Scott & Slack, Virology 46: 490, 1971.
- Shih & Kaesberg, Proc. natn. Acad. Sci. U.S.A. 70: 1799, 1973.
- Shih & Kaesberg, J. molec. Biol. 103: 77, 1976.
- Shih, Lane & Kaesberg, J. molec. Biol. 64: 353, 1972.
- Stubbs & Kaesberg, J. molec. Biol. 8: 314, 1964.
- von Wechmar & van Regenmortel, Virology 34: 36, 1968.
- Walters & Dodd, Phytopathology 59: 1055, 1969.
- Walters & Surin, Pl. Dis. Reptr 57: 833, 1973.