Details of DPV and References
DPV NO: 186 August 1978
Family: Secoviridae
Genus: Nepovirus
Species: Grapevine Bulgarian latent virus | Acronym: GBLV
Grapevine Bulgarian latent virus
G. P. Martelli Istituto di Patologia vegetale, Università di Bari, 70126 Bari, Italy
A. Quacquarelli Istituto di Patologia vegetale, Università di Bari, 70126 Bari, Italy
D. Gallitelli Istituto di Patologia vegetale, Università di Bari, 70126 Bari, Italy
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by Martelli et al. (1976, 1977).
- An RNA-containing virus with isometric particles c. 30 nm in diameter which sediment as three components. It is readily transmitted by grafting to grapevine and by inoculation of sap to a restricted range of herbaceous hosts. It has been found only in grapevines in Bulgaria and USA.
Main Diseases
Latent in some cultivars of Vitis vinifera (e.g. Rcatzitelli) (Martelli et al., 1977) although occasionally isolated from plants of cv. Julski Bisser, Cabernet Sauvignon, Cardinal and Bolgar with reduced growth and fanleaf-like symptoms (see Description No. 28) (Martelli et al., 1976). In Vitis labrusca cv. Concord the virus induces delayed budbreak, irregular elongation of the shoots, pale green foliage and straggly fruit clusters (Uyemoto et al., 1977). Rooted cuttings and seedlings of hybrid LN-33 and of several V. vinifera cultivars were symptomlessly infected following manual inoculation with Bulgarian isolates (Martelli et al., 1977).
Geographical Distribution
Reported from several Bulgarian localities and from New York State, USA.
Host Range and Symptomatology
Transmitted experimentally by inoculation of sap to a very narrow range of hosts comprising 10 species in 4 dicotyledonous families (Martelli et al., 1976, 1977; Uyemoto et al., 1977).
- Diagnostic species
- Chenopodium quinoa. In inoculated leaves,
Bulgarian isolates induce in 3-4 days local chlorotic
lesions soon turning necrotic (Fig. 1) whereas lesions
elicited by the American (NY) strain are mostly chlorotic
(Fig. 2). Systemic symptoms consist of severe leaf mottle
and necrotic flecks (Fig. 3).
- Gomphrena globosa (globe amaranth). Reddish local lesions after 6-8 days followed by distortion of non-inoculated leaves.
- Nicotiana clevelandii. A few necrotic local lesions; systemic symptoms are stunting and general leaf chlorosis.
- Gomphrena globosa (globe amaranth). Reddish local lesions after 6-8 days followed by distortion of non-inoculated leaves.
- Propagation and assay species
- Chenopodium quinoa is both a good source of virus for purification and a satisfactory local lesion host.
Strains
Naturally occurring serological variants were found in Bulgaria (Martelli et al., 1976) and USA (Uyemoto et al., 1977).
Transmission by Vectors
The NY strain was not transmitted to seedlings of C. quinoa and cucumber planted in soil infested by Xiphinema americanum, which was collected under a diseased Concord vine (Uyemoto et al., 1977).
Transmission through Seed
The NY strain is transmitted through seeds of both V. labrusca (c. 5% and C. quinoa (c. 12%) (Uyemoto et al., 1977).
Serology
Virus nucleoproteins are good immunogens yielding antisera with titres in gel-diffusion tests of up to 1/1024. Empty capsids are poorly immunogenic, their antisera having titres no higher than 1/64 (Martelli et al., 1977). In gel-diffusion tests a single precipitin band is formed. Visible precipitin bands can be obtained using undiluted crude sap of C. quinoa leaves with symptoms, or sap from infected but symptomless young grapevine leaves grown in the glasshouse or collected in the field in the spring (Martelli et al., 1976, 1977).
Relationships
Two Bulgarian isolates proved to be serologically distinguishable from each other but closely related (Martelli et al., 1976). A more distant relationship was found between Bulgarian and NY strains (Uyemoto et al., 1977; V. Savino & G. P. Martelli, unpublished information). The virus did not react with antisera to 31 viruses with isometric particles including the following members of the nepovirus group: grapevine fanleaf (5 different isolates), arabis mosaic (6 isolates), tomato black ring (5 isolates including English and Scottish strains), raspberry ringspot (3 isolates including English and type strains), tomato ringspot (6 isolates), tobacco ringspot (5 isolates), strawberry latent ringspot (4 isolates), cherry leaf roll (7 isolates, including cherry, walnut, elderberry and dogwood strains), grapevine chrome mosaic (2 isolates), artichoke Italian latent (3 isolates), peach rosette mosaic and cacao necrosis viruses (Martelli et al., 1976, 1977; Uyemoto et al., 1977).
Stability in Sap
In sap from infected C. quinoa plants infectivity is lost after dilution to 10-6, heating for 10 min at 65-70°C or storing for 15-20 days at 22°C (Martelli et al., 1977). Different Bulgarian isolates behaved similarly (Martelli et al., 1976).
Purification
Bulgarian isolates were satisfactorily purified by clarifying expressed sap of C. quinoa with 7% of magnesium-activated bentonite (Dunn & Hitchborn, 1965), followed by one cycle of differential centrifugation and sucrose density gradient centrifugation (Martelli et al., 1976, 1977). The NY strain was purified by freezing and thawing expressed sap, dialysis against ammonium sulphate (200 g/l), differential centrifugation and electrophoresis in sucrose gradients containing 0.1 ionic strength phosphate buffer, pH 7.6 (Uyemoto et al., 1977). Average yield of virus (Bulgarian isolates) is c. 15-20 A260 units/100 g of infected material (Martelli et al., 1977).
Properties of Particles
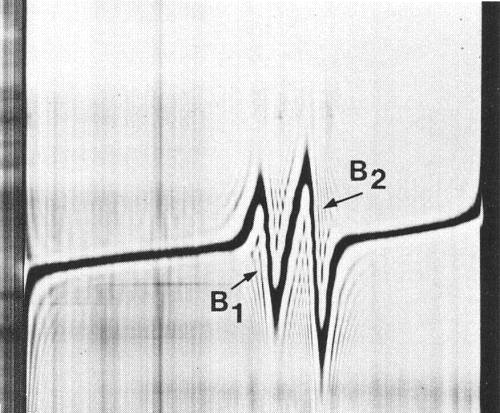
In sucrose density gradients purified virus preparations separate into two classes of particles: empty protein shells without RNA (T) sedimenting at about 52 S and nucleoprotein particles (B) sedimenting at about 123 S. In analytical ultracentrifugation (Fig. 4) and at equilibrium in CsCl (Fig. 5), B component splits into two sub-components which contain different amounts of RNA and sediment at different rates.
Sedimentation coefficients (s020,w): 52 S (T), 120 S (B1), 127 S (B2).
A260/A280 = 0.8 (T), 1.7 (B).
Buoyant densities in CsCl at 25°C (g/cm3): 1.479 (B1), 1.489 (B2).
Thermal denaturation mid-point of nucleoproteins (Tf) (Quacquarelli et al., 1976a; Piazzolla et al., 1977): 68°C.
Particle Structure
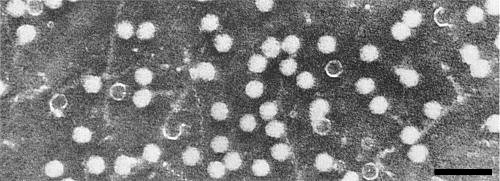
Particles are isometric, c. 30 nm in diameter, with angular outlines. T particles are penetrated by negative stain (Fig. 6).
Particle Composition
Nucleic acid: Single-stranded RNA comprising 39% (B1) and 42% (B2) of the particle weight when calculated according to Reichmann’s (1965) formula, or 39% (B1) and 40% (B2) when calculated on the basis of buoyant densities (Sehgal et al., 1970). Electrophoresis in 2.4% polyacrylamide gels in buffer (pH 7.8) containing 0.02 M sodium acetate, 0.04 M tris, 0.001 M disodium ethylene diamine tetraacetate and 0.2% sodium dodecyl sulphate separates RNA from unfractionated virus or from B particles into two components of M. Wt 2.2 and 2.1 x 106, respectively. Presumably the lighter RNA species derives from B1 particles and the heavier species from B2 particles (Martelli et al., 1977). Infective RNA is easily obtained from purified virus preparations in 0.02 M (Na-K) phosphate buffer containing 0.1 M NaCl by freezing at -25°C and thawing, or by heating at 68°C for 90 sec (Martelli et al., 1976, 1977). The single phase phenol/SDS method (Diener & Schneider, 1968) is also useful.
Protein: In 7% polyacrylamide gels containing 0.1% SDS the protein migrates as one component of M. Wt c. 54 000 (Martelli et al., 1976, 1977).
Relations with Cells and Tissues
Vines are systemically invaded. The virus occurs in high concentration in the leaves and is also isolated from roots and seeds (Martelli et al., 1977; Uyemoto et al., 1977).
Notes
The virus has biological and physico-chemical characteristics typical of nepoviruses and therefore is to be regarded as a likely member of the group. However, it is not serologically related to any of the known nepoviruses against which it has been tested and has no known vector. In its physico-chemical characteristics and hydrodynamic behaviour (e.g. two nucleoprotein components sedimenting at about the same rate and containing two RNA species with a similarly high M. Wt) grapevine Bulgarian latent virus resembles tomato ringspot virus (Schneider et al., 1974). These two viruses have been assigned to the same subgroup (Quacquarelli et al., 1976b).
Figures
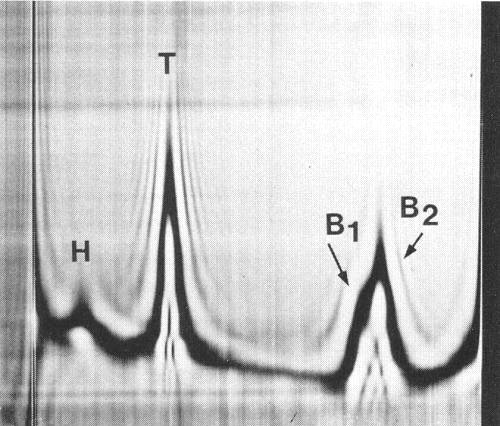
Schlieren diagram of a partially purified virus preparation after 17 min at 32 000 rev/min. T, B1 and B2 are virus-specific components whereas H is normal plant material. Component B is separating into the subcomponents B1 and B2. Sedimentation is from left to right.
References list for DPV: Grapevine Bulgarian latent virus (186)
- Diener & Schneider, Archs Biochem. Biophys. 124: 401, 1968.
- Dunn & Hitchborn, Virology 25: 171, 1965.
- Martelli, Gallitelli, Abracheva, Jankulova, Savino & Quacquarelli, Abstr. Proc. 6th Conf. int. Counc. Grapevine Viruses, Cordova 1976: 23, 1976.
- Martelli, Gallitelli, Abracheva, Savino & Quacquarelli, Ann. appl. Biol. 85: 51, 1977.
- Piazzolla, Gallitelli & Quacquarelli, J. gen. Virol. 37: 373, 1977.
- Quacquarelli, Gallitelli, Savino & Martelli, J. gen. Virol. 32: 349, 1976a.
- Quacquarelli, Gallitelli, Savino, Piazzolla & Martelli, Abstr. Proc. 6th Conf. int. Counc. Grapevine Viruses, Cordova 1976: 10, 1976b.
- Reichmann, Virology 25: 166, 1965.
- Schneider, White & Civerolo, Virology 57: 139, 1974.
- Sehgal, Jean, Bhalla, Soong & Krause, Phytopathology 60: 1778, 1970.
- Uyemoto, Taschenberg & Hummer, Pl. Dis. Reptr 61: 949, 1977.