Details of DPV and References
DPV NO: 188 August 1978
Family: Caulimoviridae
Genus: Badnavirus
Species: Rubus yellow net virus | Acronym: RYNV
Rubus yellow net virus
R. Stace-Smith Agriculture Canada Research Station, Vancouver, B.C., Canada
A. T. Jones Scottish Horticultural Research Institute, Invergowrie, Dundee, Scotland
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by
Stace-Smith (1955a).
Probable synonym
- Raspberry yellow mosaic virus (Rev. appl. Mycol. 6: 675)
-
A bacilliform virus with particles c. 80-150 x 25-31 nm. Transmitted by aphids (Amphorophora spp.) but not by mechanical inoculation of sap. Probably widespread throughout the world, usually as a complex with other viruses.
Main Diseases
When alone in red raspberry or black raspberry the virus produces a chlorosis of the tissue along the leaf veins to form a yellow net pattern (Fig. 1, Fig. 2). In red raspberry, in complex with black raspberry necrosis virus (Stace-Smith, 1955b) it causes raspberry veinbanding mosaic disease (Stace-Smith, 1956). Such diseased plants show chlorosis of the leaf lamina in areas adjacent to the main veins (Fig. 3).
Geographical Distribution
Probably worldwide, wherever Rubus cultivars are grown; usually as a complex infection with one or more other viruses.
Host Range and Symptomatology
The virus is not mechanically transmissible; it has a narrow natural host range consisting of a few species in the dicotyledonous genus Rubus. The virus has been transmitted experimentally by grafting but not by aphids to Fragaria vesca (Stace-Smith & Mellor, 1957).
-
Diagnostic species
- Rubus idaeus
(red raspberry) cvs Washington, Cuthbert and Newburgh. About 4-6 weeks after inoculation by grafting, or 3-4 weeks after inoculation by aphids, a net-like chlorosis of tissue develops along the leaf veins. In chronically infected plants, leaves may be slightly cupped downward (Fig. 1). -
R. occidentalis (black raspberry) and R. albescens. Seedlings of
these species show a diagnostic net-like chlorosis 3-4 weeks after exposure to
viruliferous aphids. The initial symptom is flecks of net-like chlorosis on the
fourth or fifth leaf beneath the tip of the infected seedling, followed by
progressively more vein chlorosis on the younger leaves. At this stage of
infection, when the young leaflets are partially expanded, chlorosis is typically
unilateral, involving one of the basal leaflets and the lower edge of the terminal
leaflet
(Fig. 2).
- Fragaria vesca (Alpine strawberry). Down-curling of young leaves about 3 weeks after graft inoculation, followed by the appearance of necrotic lesions on the petiole, wilting and death of affected leaves and, within 2 months of inoculation, death of the plant.
-
Propagation species
- R. idaeus
is suitable for maintaining cultures. All cultivars are thought to be susceptible.Assay species
- Seedlings of black raspberry (R. occidentalis) are suitable assay plants for use in aphid-transmission experiments.
Strains
Isolates from several red raspberry cultivars produced similar symptoms in black raspberry seedlings (Stace-Smith, 1956).
Transmission by Vectors
Transmitted by the raspberry aphids Amphorophora agathonica in North America and A. rubi in Europe. Other Rubus-infesting Amphorophora spp. may serve as vectors. The virus can be transmitted by the vector after an acquisition feed of 1 h but frequency of transmission is greater after a 4 h feed. There is no latent period in the vector and aphids lose the capacity to transmit the virus after feeding for 2-3 h on healthy plants (Stace-Smith, 1955a). Starved aphids may retain the virus for 1 day when held at 20°C and up to 4 days when held at 3°C (Stace-Smith, 1960).
Transmission through Seed
None reported.
Serology
No information available.
Relationships
The virus particles are similar in shape and size to those of cacao swollen shoot virus (Brunt, 1970) and to those particles associated with penyakit habang in rice (Saito et al., 1975), internal brown spot of yam (Harrison & Roberts, 1973), ‘Alomea’ disease of Colocasia (James, Kenten & Woods, 1973) and leaf spot of Dendrobium (Petzold, 1971). All these particles have been located in the vascular tissue of their natural hosts but none of those whose mode of transmission has been determined are transmitted by aphids.
Stability in Sap
Unlike the other Rubus viruses transmitted by Amphorophora spp., the virus is not inactivated in infected plants by exposure to 37°C for several weeks or months (Stace-Smith, 1960; Converse, 1966). However, by excising and rooting small tip cuttings from heat-treated plants, virus-free clones have been obtained (F. C. Mellor & R. Stace-Smith, unpublished data).
Purification
No information.
Properties of Particles
No information.
Particle Structure
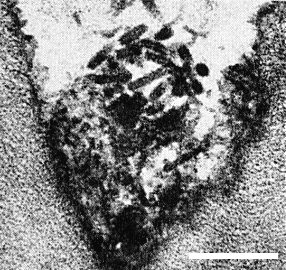
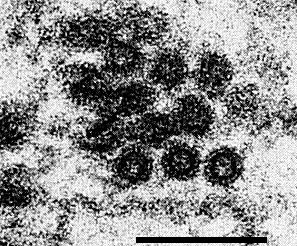
Particles are bacilliform, and in thin sections of raspberry measure 80-150 nm long and 25-31 nm wide (Jones & Roberts, 1976; Stace-Smith & Leung, 1976); both ends are rounded (Fig. 4, Fig. 6). In cross-section, particles show an electron translucent core, c. 17 nm in diameter (Fig. 7).
Particle Composition
No information.
Relations with Cells and Tissues
In black raspberry, the particles appear confined to the sieve tubes in the early stages of infection but are found in all kinds of cells including xylem parenchyma, mesophyll and epidermal cells in later stages of infection. In red raspberry, the particles were detected only in xylem parenchyma (Jones, Roberts & Murant, 1974). Particles occur singly or clustered, often in degenerated endoplasmic reticulum (Fig. 4); in clusters they lack regular orientation. In addition to virus particles, two kinds of abnormality may be seen in ultrathin sections of infected black raspberry leaf tissue (Jones & Roberts, 1976):
(1) cell wall outgrowths associated with the plasmodesmata (Fig. 5), and
(2) tubules, c. 30 nm in diameter and up to 1100 nm long aligned parallel to one another in bundles of 5 to 50 (Fig. 4).
The cell wall outgrowths and tubules are unassociated but both are more common in the leaf mesophyll cells than in vascular bundle cells.
Notes
Small bacilliform particles similar to those of rubus yellow net virus were first observed in thin sections of two red raspberry cultivars known to contain four viruses transmitted by Amphorophora rubi (Jones et al., 1974). Later work showed that these particles were present after heat treatment at 37°C and were associated with rubus yellow net virus symptoms in black raspberry (Jones & Roberts, 1976).
Of the other viruses in red raspberry that survive heat treatment at 37°C, raspberry bushy dwarf virus (Murant, 1976) and the nepoviruses (Murant, 1970) have spherical particles and raspberry vein chlorosis virus (Jones, Murant & Stace-Smith, 1977) has large bacilliform particles.
Symptoms induced by rubus yellow net virus in some red raspberry cultivars can be similar to those induced by raspberry vein chlorosis virus. However, the viruses have particles of different sizes, different aphid vectors and can be distinguished in pure culture by graft indexing to black raspberry (R. occidentalis) which is immune to raspberry vein chlorosis virus (Jones et al., 1977).
In commercial plantations several aphid-borne viruses frequently occur together in infected plants, making identification difficult. However, the detection of rubus yellow net virus in such material can be achieved either by transferring the aphid vector from field-infected plants to successive seedlings of black raspberry for chance separation of the viruses (Stace-Smith, 1956) or by maintaining plants at an air temperature of 37°C for several weeks until the other viruses are eradicated and then graft indexing to black raspberry indicators (Stace-Smith & Mellor, 1957; Jones & Roberts, 1976).
The occurrence and spread of raspberry veinbanding mosaic disease (and hence rubus yellow net virus), once common in raspberry plantations in Britain, has been restricted by the planting of stock that have been inspected and certified free from the disease. The production and maintenance of raspberry plantations free from rubus yellow net virus and the other viruses transmitted by A. rubi is now likely to be further improved by the introduction of raspberry cultivars resistant to the aphid vector (Jones, 1976).
Acknowledgements
Figs 4, 5 courtesy Scottish Horticultural Research Institute, Dundee, other figures courtesy Agriculture Canada Research Station, Vancouver.
Figures

Leaf symptoms in Rubus spp. infected with rubus yellow net virus. Systemically infected leaf of red raspberry (R. idaeus var. strigosus) cv. Washington.

Leaf symptoms in Rubus spp. infected with rubus yellow net virus. Initial systemic symptom in black raspberry (R. occidentalis).
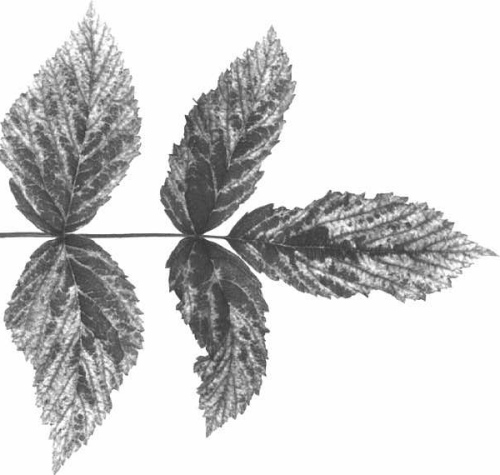
Leaf symptom of raspberry veinbanding mosaic disease induced in red raspberry cv. Cuthbert by rubus yellow net virus in complex with black raspberry necrosis virus.
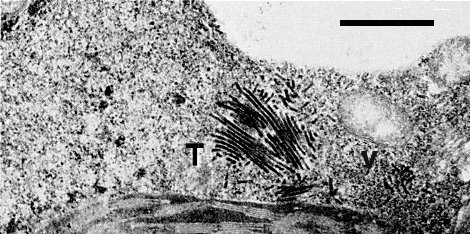
Electron micrograph of thin section of R. occidentalis infected with rubus yellow net virus: a group of tubules (T) and randomly distributed bacilliform particles (V) in a mesophyll cell. Bar represents 1 µm.

Electron micrograph of thin section of R. occidentalis infected with rubus yellow net virus: extensive cell wall outgrowths in mesophyll cells. Bar represents 1 µm.
References list for DPV: Rubus yellow net virus (188)
- Brunt, CMI/AAB Descriptions of Plant Viruses 10, 4 pp., 1970.
- Converse, Phytopathology 56: 556, 1966.
- Harrison & Roberts, Trop. Agric., Trin. 50: 335, 1973.
- James, Kenten & Woods, J. gen. Virol. 21: 145, 1973.
- Jones, Ann. appl. Biol. 82: 503, 1976.
- Jones & Roberts, Ann. appl. Biol. 84: 305, 1976.
- Jones, Roberts & Murant, Ann. appl. Biol. 77: 283, 1974.
- Jones, Murant & Stace-Smith, CMI/AAB Descriptions of Plant Viruses 174, 4 pp., 1977.
- Murant, In Virus Diseases of Small Fruits and Grapevines, p. 132, University of California Press, 1970.
- Murant, CMI/AAB Descriptions of Plant Viruses 165, 4 pp., 1976.
- Petzold, Phytopath. Z. 70: 43, 1971.
- Saito, Roechan, Tantera & Iwaki, Phytopathology 65: 793, 1975.
- Stace-Smith, Can. J. Bot. 33: 269, 1955a.
- Stace-Smith, Can. J. Bot. 33: 314, 1955b.
- Stace Smith, Can. J. Bot. 34: 435, 1956.
- Stace-Smith, Can. Pl. Dis. Surv. 40: 24, 1960.
- Stace-Smith & Leung, Proc. Am. Phytopath. Soc. 3: 320, 1976.
- Stace-Smith & Mellor, Can. J. Bot. 35: 287, 1957.