Details of DPV and References
DPV NO: 195 August 1978
Family: Alphaflexiviridae
Genus: Potexvirus
Species: Daphne virus X | Acronym: DVX
Daphne virus X
R. L. Forster Plant Diseases Division, D.S.I.R., Private Bag, Auckland, New Zealand
K. S. Milne Dept. Horticulture and Plant Health, Massey University, Palmerston North, New Zealand
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by
Forster & Milne (1975,
1978b).
A virus with flexuous filamentous particles c. 500 x 12 nm. No vector is known but the virus is spread by vegetative propagation.
Main Diseases
The virus occurs naturally in Daphne cneorum and D. odora but is not associated with symptoms in either species.
Geographical Distribution
Recorded only from New Zealand.
Host Range and Symptomatology
The virus infected 19 of 34 species in 6 of 11 dicotyledonous families (Amaranthaceae,
Chenopodiaceae, Cucurbitaceae, Leguminosae, Scrophulariaceae, Solanaceae). Only 7 species
were systemically infected (Chenopodium quinoa, Cucumis melo, Cucumis sativus, Cucurbita
maxima, Datura stramonium, Pisum sativum, Nicotiana clevelandii).
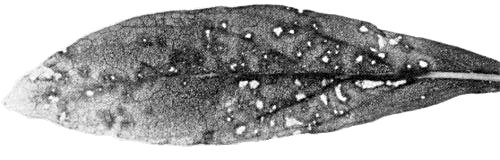
Diagnostic species
(cucumber). Sunken chlorotic local lesions in cotyledons; faint
chlorotic spotting on first systemically infected leaf, later leaves being symptomlessly
infected
(Fig. 1).
Propagation species
Assay species
Strains
None reported.
Transmission by Vectors
The virus was not transmitted from pea to pea by the aphid Myzus persicae.
Transmission through Seed
Not reported.
Serology
The virus is a good immunogen; flocculent precipitates were observed in microprecipitin tests at antiserum dilutions up to 1/2048.
Relationships
Preliminary tests (Milne & Forster, 1976) indicated a distant serological relationship to white clover mosaic virus but later experiments (Forster & Milne, 1978b) have shown no serological relationship to this virus or to clover yellow mosaic, narcissus mosaic or potato X viruses.
Stability in Sap
Infectivity was retained in N. clevelandii sap held at room temperature (c. 20°C) for 5 weeks but not for 6 weeks, in sap diluted to 10-5 but not 10-6, and after heating for 10 min at 80°C but not 85°C.
Purification
Inoculated leaves of Nicotiana clevelandii infected for 7-10 days were homogenised in 0.5 M phosphate buffer, pH 7.1, containing 0.01 M disodium ethylenediamine-tetraacetate (EDTA), 0.01 M sodium diethyldithiocarbamate (DIECA) and 0.3% 2-mercaptoethanol. Extracts were emulsified with chloroform and n-butanol and clarified by low speed centrifugation. The virus was sedimented by high speed centrifugation, resuspended in 0.05 M borate buffer, pH 8.2, containing 0.01 M EDTA and further purified by sucrose-gradient centrifugation.
Properties of Particles
The virus sediments in the analytical ultracentrifuge as a single component with a sedimentation coefficient (s°20,w) of c. 110 S.
A260/A280: 1.22.
Buoyant density: 1.28 g/cm3 in CsCl.
Particle Structure
Particles are flexuous filaments c. 500 x 12 nm (Fig. 2). In uranyl acetate many particles show distinct cross-banding.
Particle Composition
Nucleic acid: c. 6% of particle weight (estimated spectrophotometrically).
Protein: In limited tests using polyacrylamide gel electrophoresis with sodium dodecyl sulphate, a single polypeptide was observed with M. Wt c. 23,000.
Relations with Cells and Tissues
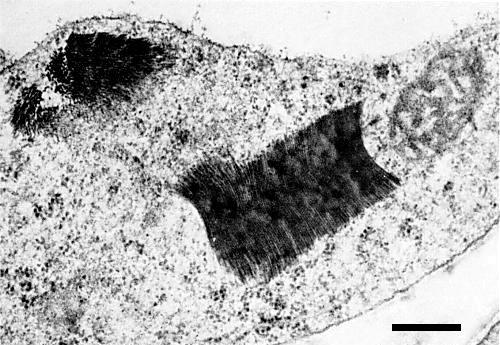
In ultrathin sections of systemically infected pea leaflets, virus-like particles were observed in large aggregates (Fig. 4) or as unaggregated particles scattered through the cytoplasm.
Notes
In New Zealand, no D. cneorum plants have been found free of daphne virus X out of more than 100 tested. The virus has properties typical of members of the potexvirus group, but can be distinguished from other members of the group by host range, symptomatology and serology. Only two other viruses infecting daphne have flexuous filamentous particles (Forster & Milne, 1975); daphne virus S has particles c. 720 nm long (Forster & Milne, 1978a), and daphne virus Y has particles c. 730 nm long (Forster & Milne, 1976). Unlike daphne virus X, these viruses do not infect Cucumis sativus or Gomphrena globosa. None of the other viruses recorded in daphne give a range of symptoms resembling those of daphne virus X on the species listed under ‘Diagnostic hosts’.
Figures
References list for DPV: Daphne virus X (195)
- Forster & Milne, N. Z. Jl agric. Res. 18: 391, 1975.
- Forster & Milne, N. Z. Jl agric. Res. 19: 359, 1976.
- Forster & Milne, N. Z. Jl agric. Res. 21: 131, 1978a.
- Forster & Milne, N. Z. Jl agric. Res. 21: 137, 1978b.
- Milne & Forster, Acta Hort. 59: 95, 1976.