Details of DPV and References
DPV NO: 197 August 1978
Family: Secoviridae
Genus: Comovirus
Species: Cowpea mosaic virus | Acronym: CPMV
There is a more recent description of this virus: DPV 378.
This is a revised version of DPV 47
Cowpea mosaic virus
A. van Kammen Department of Molecular Biology, Agricultural University, Wageningen, The Netherlands
C. P. de Jager Department of Virology, Agricultural University, Wageningen, The Netherlands
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by
Chant (1959) and
Van Hoof (1963).
Synonyms
- Cowpea yellow mosaic virus (Rev. appl. Mycol. 39: 204)
- Cowpea mosaic virus, yellow strain (Rev. appl. Mycol. 43: 2773; 53: 2381)
-
An RNA-containing virus with isometric particles about 24 nm in diameter. It has a limited host range, is transmitted mainly by beetles and readily by sap inoculation. Infected plants contain two kinds of nucleoprotein particle similar in size but differing in RNA content. Particles containing no RNA are also produced by most isolates. The RNA species in different particle types represent separate parts of the viral genome.
Main Diseases
Causes mosaic diseases in Vigna spp. Leaf area and flower production are decreased. Yield reductions up to 95% have been reported. Late infections had less effect on yield than early ones (Chant, 1960). Bock (1971) reports the susceptibility of pigeon pea (Cajanus cajan) which is widely grown in the coastal areas of Kenya as a perennial crop and may serve as a reservoir of the virus.
Geographical Distribution
Reported from Nigeria, Kenya, Surinam, Cuba and USA.
Host Range and Symptomatology
The host range is rather limited; few hosts are known outside the Leguminosae. Almost all host species show necrotic or chlorotic lesions in inoculated leaves. Cowpea varieties differ greatly in type of reaction and severity of symptoms. Immunity, tolerance and hypersensitivity occur. Symptoms in susceptible varieties may range from a hardly discernible green mottle to distinct yellow mosaic and distortion with significantly reduced growth (Bliss & Robertson, 1971).
-
Diagnostic species
- Vigna unguiculata
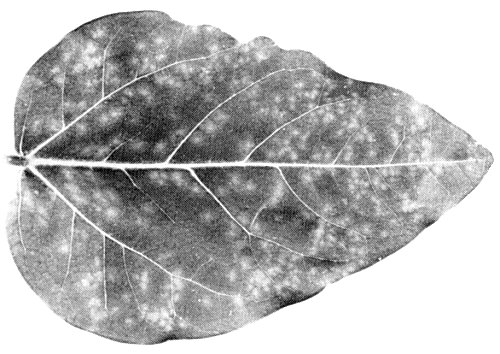
(cowpea) cv. Blackeye Early Ramshorn. Chlorotic spots with diffuse borders (diam. 1-3 mm) are produced in inoculated primary leaves (Fig. 1). Trifoliolate leaves develop a bright yellow or light green mosaic of increasing severity in younger leaves. Distortion and reduction in size are moderate (Fig. 2). Plants do not show necrosis. - Chenopodium amaranticolor. In inoculated leaves, yellow local lesions (diam. 0.5-1 mm) later becoming necrotic; systemic symptoms are severe mosaic, chlorotic spots, distortion and puckering (Fig. 3).
-
Propagation species
- Vigna unguiculata
cv. Blackeye Early Ramshorn is a good source of virus for purification and for maintaining cultures.Assay species
- Phaseolus vulgaris
cv. Pinto and Chenopodium amaranticolor are suitable local lesion hosts. For local lesion transfer Pinto beans should preferably be used because this host is not systemically infected and the lesions have a high virus content.
Strains
Chant (1962) mentioned the possibility that beetle-transmitted cowpea viruses from Trinidad (Dale, 1949) and Nigeria (Chant, 1959) were related strains. Agrawal (1964) classified five isolates into two strains and designated these as yellow and severe strains. A Nigerian isolate and the isolate SB from Surinam (collected at Santo Boma; Van Hoof, 1963) belonged to the yellow strain. A Trinidad isolate and two other isolates from Surinam represented the severe strain. Both strains were included in the previous Description of cowpea mosaic virus (Van Kammen, 1971). However, Swaans & Van Kammen (1973), having made a detailed comparison of the two strains, proposed that they be regarded as two different viruses of the cowpea mosaic virus group and this revised Description now deals only with the yellow strain. Because the SB isolate is designated as the type member of the comovirus group (Fenner, 1976), the name cowpea mosaic virus is maintained for the yellow strain. The severe strain will be described separately, as cowpea severe mosaic virus.
Nigerian and SB isolates do not seem to differ in host range and appear to be serologically identical or very similar (Agrawal & Maat, 1964). The same holds for the isolate from the USA and the SB-isolate (McLaughlin et al., 1977). Although there are some differences between host ranges of the Nigerian and the Kenyan isolates they are closely related serologically (Bock, 1971). The Cuban isolate (Kvicala, Smrz & Blanco, 1970) also has a different host range but was not tested serologically. However, because it infects Chenopodium amaranticolor systemically it should probably be grouped together with the above mentioned isolates of the former yellow strain. In the laboratory Bruening (1969) isolated from the SB-strain two naturally occurring variants which differed from the parent strain in the relative amount of RNA-free particles produced. De Jager (1973) isolated from the SB-strain two naturally occurring variants which, unlike the parent strain, infected Early Red cowpeas systemically. By treatment of virus particles or extracted RNA with nitrous acid, mutants may readily be obtained. Most of these mutants are characterized by less conspicuous symptoms and/or decreased virus production (De Jager & Van Kammen, 1970; De Jager, 1976; De Jager et al., 1977). Siler, Babcock & Bruening (1976) reported the isolation, after bisulphite treatment, of a mutant with greater specific infectivity.
Transmission by Vectors
Transmitted by various beetles with biting mouthparts. In Africa the chrysomelid beetle Ootheca mutabilis is an efficient vector (Chant, 1959; Bock, 1971) but Paraluperodes quaternus (Chrysomelidae) and Nematocerus acerbus (Curculionidae) were also found to transmit the virus (Whitney & Gilmer, 1974). In Surinam and Cuba respectively, Ceratoma variegata and C. ruciformis are incriminated as vectors (Van Hoof, 1963; Kvicala et al., 1970). Jansen & Staples (1971) listed C. trifurcata, Diabrotica balteata, D. undecimpunctata howardi, D. virgifera and Acalymma vittatum (all chrysomelid beetles) as vectors. Beetle vectors may remain viruliferous for 1-2 to more than 8 days (Chant, 1959; Jansen & Staples, 1971). Transmission efficiency and retention of infectivity are correlated with the amount of vector feeding (Jansen & Staples, 1971). Virus infectivity was found in excrements of beetles (Kvicala et al., 1970). Whitney & Gilmer (1974) reported also transmission by two species of thrips (Sericothrips occipitalis and Taeniothrips sjostedti) and by two species of grasshoppers (Cantotops spissus spissus and Zonocerus variegatus).
Transmission through Seed
Gilmer, Whitney & Williams (1974) reported 1-5% seed transmission in Nigeria.
Serology
The virus is strongly immunogenic. Standard methods using rabbits give antisera with titres of 1/1024 in the Ouchterlony double diffusion test, but higher titres may be obtained (Agrawal & Maat, 1964). Virus preparations tested by the Ouchterlony method give a single band of precipitate, even though they contain more than one electrophoretic component.
Relationships
Little systematic work has been done to compare isolates of the virus from different parts of the world. However, isolates from Surinam (SB-isolate), Nigeria, Kenya and USA are closely related serologically. A weak serological relationship is reported between cowpea mosaic virus as described here and some other viruses of the comovirus group i.e. cowpea severe mosaic virus (formerly known as the severe strain of cowpea mosaic virus; Swaans & Van Kammen, 1973); bean pod mottle virus (Bancroft, cited by Agrawal & Maat, 1964); red clover mottle virus (Agrawal, 1964); broad bean true mosaic virus (Jones & Barker, 1976); bean rugose mosaic virus (Gamez, 1972).
Stability in Sap
Properties in vitro reported for the various isolates vary considerably, probably because of the use of different source plants and/or assay hosts. Dilution end-points range from 10-4.7 to 10-6.7. Thermal inactivation points were reported from 55 to 65°C. Longevity in vitro was found to vary from 4 to 10 days.
Purification
Yields of virus may reach 2 g/kg leaf tissue from Vigna plants grown at 30°C in a growth chamber. Freshly harvested leaves are homogenized with twice their weight of 0. 1 M phosphate buffer, pH 7.0. The homogenate is squeezed through two layers of Miracloth and centrifuged at 15,000 g for 20 min. The supernatant fluid is kept, and the pellet is washed once by suspending in phosphate buffer (0.25 ml/g leaf tissue) followed by centrifugation at 15,000 g. The combined supernatant fluids are stirred for 1 min with 0.7 volume of a 1:1 mixture of chloroform and n-butanol. After low speed centrifugation, the clear aqueous layer is removed, and the virus precipitated by adding polyethylene glycol 6000 (PEG) to 4% and NaCl to 0.2 M, and stirring for 60 min at room temperature. The precipitate after centrifugation at 15,000 g for 15 min is suspended in phosphate buffer (0.5 ml/g leaf tissue). After centrifugation at 27,000 g for 15 min, the supernatant fluid containing the virus is kept and the pellet is extracted once more in a few ml of phosphate buffer and then centrifuged at 27,000 g for 15 min. The combined supernatant fluids are layered on top of 1 ml of 40% (w/v) sucrose in 0.1 M phosphate buffer in a centrifuge tube and then centrifuged at 150,000 g for 2.5 h. The clear virus pellet is dissolved in sterile double-distilled water and centrifuged at 10,000 g for 15 min to remove possible contaminants. This procedure (Klootwijk et al., 1977) provides highly purified virus. It is a combination of the older methods of purification using chloroform/butanol mixtures (Bruening & Agrawal, 1967) or PEG and NaCl (Van Kammen, 1967).
Properties of Particles
Purified preparations of virus contain three centrifugal components: empty protein shells without RNA (T) and two nucleoprotein components (M and B), containing 25% and 36% RNA respectively. The separated nucleoprotein components are not infective (Van Kammen, 1968), but mixtures of the M and B components are. The infectivity of a mixture depends upon the proportions of the two components and the concentration of the component present in the lowest amount. Mixtures of the M or B components with the B or M components of mutants of cowpea mosaic virus are infective (Bruening, 1969; De Jager & Van Kammen, 1970; De Jager, 1976) but heterologous mixtures of components of cowpea mosaic virus and those of other comoviruses are not.
Sedimentation coefficients (s20,w) at infinite dilution (svedbergs): 58 (T), 95 (M), 115 (B). The proportion of M:B is about 1:1.
Molecular weight: 3.80 x 106 (T), 5.15 x 106 (M) and 5.87 x 106 (B).
Isoelectric point: between pH 3.7 and 4.5.
Absorbances at 260 nm (1 mg/ml, 1 cm light path): 6.2 (M), 10.0 (B), at 280 nm (1 mg/ml, 1 cm light path): 1.28 (T).
A260/A280: 1.64 (unfractionated virus), 1.61 (M), 1.74 (B).
Buoyant density in CsCl (g/ml): 1.288 (T), 1.395 (M); component B gives two bands of density 1.406 and 1.447. The two bands have the same sedimentation coefficient (Bruening, 1969; Geelen, 1974).
Purified preparations of the virus contain two electrophoretic components, each of which contains all three centrifugal components (Semancik, 1966). The electrophoretic forms can be separated by electrophoresis in a sucrose gradient. Electrophoretic mobility: -4.0 to -4.25 x 10-5 and -2.6 to -2.8 x 10-5 cm2 sec-1 volt-1 in 0.1 M phosphate buffer. The slower migrating form predominates in early infection and the faster one in late infection. The slower migrating form is converted into the faster migrating form in vivo by loss of a peptide with a M. Wt of approximately 2500 from the smaller capsid protein. The conversion of the slower form into the faster form can be achieved in vitro by incubation with proteolytic enzymes. The two electrophoretic forms of the virus have similar infectivity (Niblett & Semancik, 1969; Geelen, Rezelman & Van Kammen, 1973).
Particle Structure
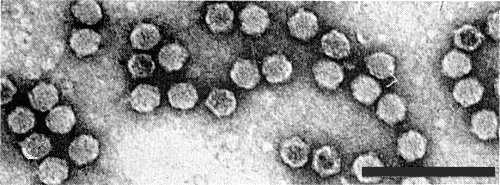
Particles are icosahedral with 5:3:2 axial symmetry, and a diameter of 20-24 nm (Fig. 4). By three dimensional image reconstruction of electron micrographs of particles negatively stained with uranyl acetate a model is proposed consisting of twelve pentamers of the larger coat protein at the 5-fold axes and twenty trimers of the smaller coat protein at the 3-fold axes (Crowther, Geelen & Mellema, 1974). In this model the protein shell consists of 60 subunits each composed of the two structural proteins arranged in interpenetrating T = 1 lattices.
Particle Composition
Nucleic acid: RNA, single-stranded. Components M and B each have a single RNA molecule with sedimentation coefficients (s20,w) of 26 S and 34 S and M. Wt of 1.37 x 106 and 2.02 x 106, respectively (Reijnders et al., 1974). The base compositions (molar percentages of nucleotides) are, respectively, G 20.7; A 28.4; C 19.3; U 31.6 and G 22.9; A 28.5; C 17.2; U 31.4 (Van Kammen & Van Griensven, 1970). Both RNA strands contain a sequence of polyadenylic acid 150-200 residues long at their 3'-end (El Manna & Bruening, 1973). The two RNAs have no m7G(5')ppp(5')N...., a structure referred to as a cap, found with many plant viral RNA species, or (p)(p)pN . . ., at their 5'-ends (Klootwijk et al., 1977).
In recombination tests with wild and mutant strains the 26 S RNA was shown to contain the gene specifying the smaller capsid protein (Gopo & Frist, 1977) and to be responsible for the relative amount of T-component produced (Bruening, 1969; De Jager & Van Kammen, 1970). The 34 S RNA seemed to control the rate of conversion of the slower into the faster electrophoretic form and the specific infectivity (Siler et al., 1976). Mutations in both RNA species were found to affect local and systemic symptoms and systemic spread of the virus in beans and cowpeas (De Jager & Van Kammen, 1970; De Jager, 1973; 1976). A mutation causing temperature sensitivity was located in the 26 S RNA (De Jager et al., 1977).
Protein: The protein shell of the virus consists of two proteins of M. Wt c. 37,000 and 22,000 in equimolar amounts (Wu & Bruening, 1971; Geelen, Van Kammen & Verduin, 1972). The coat protein contains about 1.9% carbohydrates covalently linked (Partridge et al., 1974).
Polyamines: Purified virus particles contain 5.05 µg spermidine and 0.17 µg spermine per mg virus (Bruening, El Manna & Wu, 1968; Nickerson & Lane, 1977).
Relations with Cells and Tissues
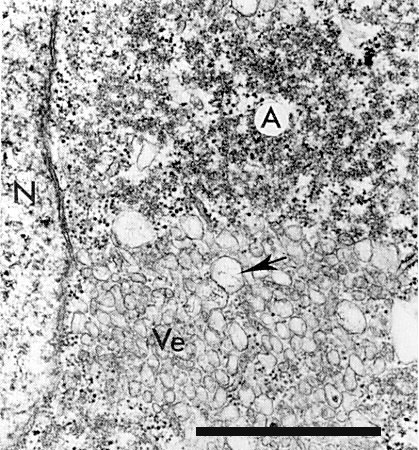
The virus particles occur scattered and in clusters throughout the cytoplasm. They do not form crystalline arrays. Inclusion bodies can be observed in virus-infected cells by the light microscope, after staining with phloxine, as a red amorphous mass near or surrounding the nucleus (Agrawal, 1964; Swaans & Van Kammen, 1973). Electron micrographs of virus-infected cells show cytopathic structures, often adjacent to the nucleus (Fig. 5), consisting of arrays of vesicles forming a kind of reticulum, the spaces between the vesicle areas being filled with electron-dense material that does not seem to have obvious structure. Virus particles can be seen embedded in this material. The vesicles often contain fibrillar material of unknown nature (De Zoeten, Assink & Van Kammen, 1974). The replication of viral RNA is closely associated with the membranes of the vesicles of the cytopathic structures as shown by fractionation of homogenates of virus-infected cells and autoradiography (Assink, Swaans & Van Kammen, 1973; De Zoeten et al., 1974). Infected Vigna leaves contain a RNA-dependent RNA polymerase which is closely bound to cytoplasmic membranes and is able to synthesize viral RNA in vitro (Zabel, Weenen-Swaans & Van Kammen, 1974). Similar cytopathic structures are found in Vigna mesophyll protoplasts infected with cowpea mosaic virus, but not in uninfected protoplasts (Hibi, Rezelman & Van Kammen, 1975).
Notes
The cowpea mosaic virus described here is the type member of the comovirus group (Fenner, 1976). Besides the viruses mentioned by Fenner this group includes pea green mottle virus (Valenta et al., 1969), pea mild mosaic virus (Clark, 1972), Andean potato mottle virus (Fribourg, Jones & Koenig, 1977) and cowpea severe mosaic virus (formerly called the severe strain of cowpea mosaic virus). Many viruses from other groups have been isolated from Vigna spp. showing mosaic symptoms. The most notable of these are: cowpea aphid-borne mosaic virus (Bock & Conti, 1974); cowpea chlorotic mottle virus (Bancroft, 1971); cowpea mild mottle virus (Brunt & Kenten, 1974); sunnhemp mosaic virus (Kassanis & Varma, 1975); a strain of southern bean mosaic virus (Shepherd, 1971); a strain of bean common mosaic virus (Sachchidananda et al., 1973). Cowpea mosaic virus may be distinguished from these and other viruses of cowpea by the morphology of its particles and their behaviour in the ultracentrifuge and by its antigenic properties.
Because of the small plots used for cowpea growing and the extreme prevalence of the beetle vector, the use of insecticides for vector control is not practicable. Use of resistant cultivars offers the best means of disease control.
Figures
References list for DPV: Cowpea mosaic virus (197)
- Agrawal, Meded. LandbwHogesch. Wageningen 64 (5): 53 pp., 1964.
- Agrawal & Maat, Nature, Lond., 202: 674, 1964.
- Assink, Swaans & Van Kammen, Virology 53: 384, 1973.
- Bancroft, CMI/AAB Descriptions of Plant Viruses 49, 4 pp., 1971.
- Bliss & Robertson, Crop Science 11: 258, 1971.
- Bock, E. Afr. agric. For. J. 37: 60, 1971.
- Bock & Conti, CMI/AAB Descriptions of Plant Viruses 134, 4 pp., 1974.
- Bruening, Virology 37: 577, 1969.
- Bruening & Agrawal, Virology 32: 306, 1967.
- Bruening, El Manna & Wu, Fed. Proc. 27: 794, 1968.
- Brunt & Kenten, CMI/AAB Descriptions of Plant Viruses 140, 4 pp., 1974.
- Chant, Ann. appl. Biol. 47: 565, 1959.
- Chant, Emp. J. exp. Agric. 28: 114, 1960.
- Chant, Ann. appl. Biol. 50: 159, 1962.
- Clark, N. Z. Jl. agric. Res. 15: 846, 1972.
- Crowther, Geelen & Mellema, Virology 57: 20, 1974.
- Dale, Ann. appl. Biol. 36: 327, 1949.
- De Jager, Abstr. 2nd int. Congr. Plant Path., no. 257, 1973.
- De Jager, Virology 70: 151, 1976.
- De Jager & Van Kammen, Virology 41: 281, 1970.
- De Jager, Zabel, Van der Beek & Van Kammen, Virology 76: 164, 1977.
- De Zoeten, Assink & Van Kammen, Virology 59: 341, 1974.
- El Manna & Bruening, Virology 56: 198, 1973.
- Fenner, Intervirology 7: 1, 1976.
- Fribourg, Jones & Koenig, Phytopathology 67: 969, 1977.
- Gamez, Turrialba 22: 249, 1972.
- Geelen, Doctoral Thesis, Agric. Univ. Wageningen, 1974.
- Geelen, Rezelman & Van Kammen, Virology 51: 279, 1973.
- Geelen, Van Kammen & Verduin, Virology 49: 205, 1972.
- Gilmer, Whitney & Williams, Proc. 1st IITA Grain LegumeImprovement Workshop, Ibadan, Nigeria, 325 pp., 1974.
- Gopo & Frist, Virology 79: 259, 1977.
- Hibi, Rezelman & Van Kammen, Virology 64: 308, 1975.
- Jansen & Staples, J. econ. Ent. 64: 365, 1971.
- Jones & Barker, Ann. appl. Biol. 83: 231, 1976.
- Kassanis & Varma, CMI/AAB Descriptions of Plant Viruses 153, 4 pp., 1975.
- Klootwijk, Klein, Zabel & Van Kammen, Cell 11: 73, 1977.
- Kvicala, Smrz & Blanco, Phytopath. Z. 69: 223, 1970.
- McLaughlin, Thongmeearkom, Milbrath & Goodman, Phytopathology 67: 844, 1977.
- Niblett & Semancik, Virology 38: 685, 1969.
- Nickerson & Lane, Virology 81: 455, 1977.
- Partridge, Shannon, Gumpf & Colbaugh, Nature, Lond. 247: 391, 1974.
- Reijnders, Aalbers, Van Kammen & Thuring, Virology 60: 515, 1974.
- Sachchidananda, Singh, Prakash & Verma, Z. PflKrankh. PflPath. PflSchutz 80: 88, 1973.
- Semancik, Virology 30: 689, 1966.
- Shepherd, CMI/AAB Descriptions of Plant Viruses 57, 4 pp., 1971.
- Siler, Babcock & Bruening, Virology 71: 560, 1976.
- Swaans & Van Kammen, Neth. J. Pl. Path. 79: 257, 1973.
- Valenta, Gressnerová, Marcinka & Nermut, Acta Virol. 13: 422, 1969.
- Van Hoof, Surin. Landb. 11: 131, 1963.
- Van Kammen, CMI/AAB Descriptions of Plant Viruses 47, 4 pp., 1971.
- Van Kammen, Virology 31: 633, 1967.
- Van Kammen, Virology 34: 312, 1968.
- Van Kammen & Van Griensven, Virology 41: 274, 1970.
- Whitney & Gilmer, Ann. appl. Biol. 77: 17, 1974.
- Wu & Bruening, Virology 46: 596, 1971.
- Zabel, Weenen-Swaans & Van Kammen, J. Virol. 14: 1049, 1974.