Details of DPV and References
DPV NO: 201 July 1979
Family: Bromoviridae
Genus: Ilarvirus
Species: Lilac ring mottle virus | Acronym: LiRMoV
Lilac ring mottle virus
F. A. van der Meer Research Institute for Plant Protection, Binnenhaven 12, P.O. Box 42, 6700 AA Wageningen, Netherlands
H. Huttinga Research Institute for Plant Protection, Binnenhaven 12, P.O. Box 42, 6700 AA Wageningen, Netherlands
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
Described by Van der Meer, Huttinga & Maat (1976).
A virus with isometric, RNA-containing particles about 27 nm in diameter, which sediment as two components. Detected only in lilac from which it is readily transmissible by inoculation of sap to several herbaceous hosts. Seed-borne in some herbaceous hosts. Vector unknown. Reported only from the Netherlands.
Main Diseases
Causes rings and line patterns on lilac (Syringa vulgaris) (Fig. 1); these, however, cannot be distinguished from symptoms caused by other viruses of lilac (Van der Meer, 1976; 1978).
Geographical Distribution
Reported only from the Netherlands.
Host Range and Symptomatology
Host range seems to be fairly wide although not many species have been tested. Besides lilac, species of Chenopodiaceae, Amaranthaceae, Leguminosae, Solanaceae and Apocynaceae are susceptible ( Van der Meer et al., 1976) Readily transmissible by inoculation of sap.
-
Diagnostic species
- Celosia argentea.
Faint chlorotic lesions followed by a bright systemic chlorosis. - Chenopodium quinoa. Systemic epinasty after 6 to 20 days followed by
severe stunting
(Fig. 2);
often there is tip necrosis.
- Nicotiana tabacum (tobacco), type White Burley, and N. glutinosa. Transient wilting of tip leaves (Fig. 3).
-
Propagation species
- Chenopodium quinoa.
Assay species
- The virus may be assayed by recording systemic infections in whole plants of Chenopodium quinoa. No local lesion host is known.
Strains
Isolates differing in the severity of symptoms induced in herbaceous hosts have been noted. No serological differences have been detected.
Transmission by Vectors
No vectors are known. Not transmitted by Myzus persicae (Van der Meer et al., 1976).
Transmission through Seed
No seed transmission was detected in lilac (Van der Meer, 1976). However, seeds from infected plants of Chenopodium quinoa, Chenopodium amaranticolor and Celosia argentea gave rise to 13, 91 and 24% infected plants, respectively (Van der Meer et al., 1976).
Serology
Strongly immunogenic in rabbits. An antiserum with a titre of 1/1024 has been prepared. In the Ouchterlony double diffusion test the antiserum reacted with virus preparations purified from Chenopodium quinoa and also with crude extracts of C. quinoa and lilac (Van der Meer et al., 1976).
Relationships
The properties, structure and composition of its particles indicate that lilac ring mottle virus belongs to the ilarvirus group, but no serological relationship was detected to four members of this group, namely apple mosaic, elm mottle, prunus necrotic ringspot and tobacco streak viruses.
Stability in Sap
In Chenopodium quinoa sap, the infectivity dilution end-point is about 10-4, the thermal inactivation point (10 min) is about 63°C, and infectivity is retained for 3 to 5 h at 20°C.
Purification
Harvest leaves from infected Chenopodium quinoa with severe symptoms and store overnight at 4°C. Disrupt 100 g portions of leaf in a blender with a mixture of 35 ml carbon tetrachloride, 35 ml chloroform, 15 ml diethyl ether and 270 ml 0.018 M phosphate-citric acid buffer, pH 7, containing 0.1% thioglycollic acid. Centrifuge the homogenate at low speed. Concentrate the virus from the upper phase by precipitation with 8% (w/v) polyethylene glycol M. Wt 6000 and two cycles of differential centrifugation. Purify further by rate zonal centrifugation in sucrose gradients, or, after fixation with 2% glutaraldehyde, isopycnic centrifugation in CsCl or Cs2SO4. Throughout the purification keep the virus in 0.018 M phosphate-citric acid buffer, pH 7 (Van der Meer et al., 1976).
Properties of Particles
Purified preparations contain two centrifugal components (Fig. 4), top (T) and bottom (B). B is infective, T is not, but it can slightly enhance the infectivity of B (Van der Meer et al., 1976).
Sedimentation coefficients (in 0.018 M phosphate-citric acid, pH 7, at 20°C and at infinite dilution) (svedbergs): 83 (T) and 98 (B).
Buoyant densities of T and B are the same: 1.345 g/cm3 in CsCl and 1.265 g/cm3 in Cs2SO4.
Electrophoresis in 2.6% polyacrylamide gels separates the virus into a faster
moving band, corresponding with T, and a slower moving one corresponding with B
(Van der Meer et al., 1976;
Huttinga & Mosch, 1976).
A260/A280: 1.43 to 1.46 for preparations containing both components, or either alone.
Particle Structure
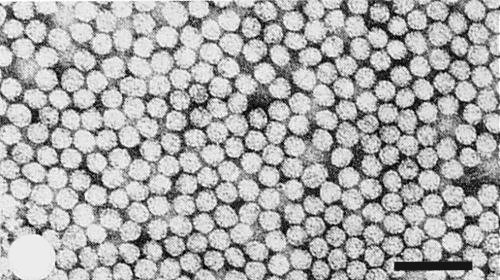
Particles are very sensitive to buffers of high ionic strength and must be fixed with glutaraldehyde before staining with phosphotungstate for electron microscopy. The virus then appears as irregularly shaped isometric particles with some heterogeneity in size (Fig. 5). Average particle diameter is 27 nm (Van der Meer et al., 1976).
Particle Composition
Nucleic acid: RNA, single-stranded. Based on A260/A280 ratios the amount of RNA in the virus particles is estimated to be 13-14%. Unfractionated preparations contain RNA species with M. Wt, estimated by polyacrylamide gel electrophoresis, of 1.18 x 106 (RNA-1), 1.13 x 106 (RNA-2), 0.90 x 106 (RNA-3) and 0.37 x 106 (RNA-4) (H. Huttinga & W. H. M. Mosch, unpublished data). T contains only RNA-3 and RNA-4; B contains predominantly RNA-1 and RNA-2, but also a small amount of RNA-4 and some RNA-3. All four RNA species are necessary for infection, but RNA-4 can be replaced by the coat protein (Huttinga & Mosch, 1976).
Protein: T and B particles contain a single species of polypeptide, of M. Wt 27,500 (Huttinga & Mosch, 1976).
Relations with Cells and Tissues
No information.
Notes
By back transmission from herbaceous hosts to lilac seedlings, it has been shown that at least four viruses detected in lilac, namely arabis mosaic, lilac ring mottle, tobacco rattle (Van der Meer, 1976) and elm mottle (Schmelzer, 1969), cause rings and/or line patterns in lilac. It is not known whether five other viruses detected in lilac, cherry leaf roll, tomato bushy stunt (Novak, 1977), tomato black ring (Schmelzer, 1970; Van der Meer, 1978), lilac chlorotic leaf spot (Brunt, 1978) and strawberry latent ringspot (Van der Meer, 1976), can cause similar symptoms. However, from many lilac plants with rings and line patterns on the leaves, none of these nine readily sap-transmissible viruses could be isolated (Schmelzer, 1970; Van der Meer, 1976; Novak, 1977), suggesting that at least one other virus causes rings and line patterns in lilac. Lilac ring mottle virus is therefore identified only by transmission to herbaceous indicators and by serological tests.
Figures
References list for DPV: Lilac ring mottle virus (201)
- Brunt, Ann. appl. Biol 88: 383, 1978.
- Huttinga & Mosch, Acta Hort. 59: 113, 1976.
- Novak, Biologia Pl. 19: 264, 1977.
- Schmelzer, Phytopath. Z. 64: 39, 1969.
- Schmelzer, Phytopath. Z. 67: 285, 1970.
- Van der Meer, Acta Hort. 59: 105, 1976.
- Van der Meer, Jaarb. Proefstn. Boomkwek. Boskoop, 1977: 73, 1978.
- Van der Meer, Huttinga & Maat, Neth. J. Pl. Path. 82: 67, 1976.