Details of DPV and References
DPV NO: 206 July 1979
Family: Secoviridae
Genus: Nepovirus
Species: Potato black ringspot virus | Acronym: PBRSV
Potato black ringspot virus
L. F. Salazar International Potato Center, Apartado 5969, Lima, Peru
B. D. Harrison Scottish Horticultural Research Institute, Invergowrie, Dundee DD2 5DA, Scotland
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by Salazar & Harrison (1977, 1978a).
- Synonym
- Potato calico strain of tobacco ringspot virus
(Rev. Pl. Path. 56, 5153).
- A virus with particles c. 25 nm in diameter, sedimenting as three components, and with a RNA genome in two pieces. It has a wide host range, is readily transmitted by inoculation with sap and occurs in Peru.
Main Diseases
Causes systemic necrotic spotting in foliage of potato (Solanum tuberosum) (Salazar & Harrison, 1977). The potato calico strain is associated with an intense generalized yellowing of the leaves in some cultivars, notably cv. Ticahuasi (Fribourg, 1977).
Geographical Distribution
Recorded only from Peru (Salazar, 1972).
Host Range and Symptomatology
Host range is wide; 40 out of 41 species inoculated with sap were infected. Hosts include species in 11 dicotyledonous families (Salazar & Harrison, 1978a).
- Diagnostic species
- Chenopodium amaranticolor and C. quinoa.
Necrotic lesions in inoculated leaves; systemic necrosis
develops about 6 to 8 days after inoculation and young plants
may be killed (Fig. 2).
- Beta vulgaris (sugar beet). Necrotic spots and rings are produced in inoculated leaves (Fig. 5). The first systemically infected leaves develop chlorotic or necrotic ringspots; leaves produced later are less obviously affected.
- Lycopersicon esculentum (tomato) cv. Kondine Red. No symptoms in inoculated leaves. Necrotic spots, rings or line patterns are produced in systemically infected leaves 15 to 20 days after inoculation (Fig. 3). See also under Strains.
- Nicotiana tabacum White Burley (tobacco). No symptoms or a few small necrotic spots in inoculated leaves. Systemically infected leaves develop necrotic ringspots and line patterns (Fig. 4) and young plants may be killed.
- Solanum tuberosum ssp. tuberosum (potato) cvs Arran Pilot and Maris Bard. Necrotic spots in inoculated leaves. The first systemic symptoms in plants infected by grafting or by inoculation with sap are necrotic ringspots (Fig. 1). The virus is passed efficiently through tubers to progeny plants; most of these have no obvious leaf symptoms but a few may develop systemic necrotic spotting or vein necrosis.
- Beta vulgaris (sugar beet). Necrotic spots and rings are produced in inoculated leaves (Fig. 5). The first systemically infected leaves develop chlorotic or necrotic ringspots; leaves produced later are less obviously affected.
- Propagation species
- Nicotiana clevelandii is suitable as a source of virus
for purification.
- Assay species
- Chenopodium amaranticolor and C. quinoa are suitable for local lesion assay.
Strains
Potato calico strain of Fribourg (1977). First described as a strain of tobacco ringspot virus, this isolate differs only slightly in antigenic constitution from the type strain of potato black ringspot virus and the two could not be distinguished by plant-protection tests in Nicotiana angustifolia (Salazar & Harrison, 1978b). Reported not to infect Lycopersicon esculentum, and not to induce systemic symptoms in Cyamopsis tetragonoloba and Phaseolus vulgaris (Fribourg, 1977).
Transmission by Vectors
None known; not transmitted by the nematode Longidorus elongatus or the aphid Myzus persicae (Salazar & Harrison, 1978a).
Transmission through Seed
Not detected in Datura stramonium, Solanum demissum ‘A’ or Capsicum annuum (L. F. Salazar & B. D. Harrison, unpublished results).
Serology
Strongly immunogenic in rabbits. Injection of a total of 5 mg virus resulted in antiserum with a precipitin titre of 1/2000. Virus in sap or purified preparations can be detected by double diffusion tests in agar or agarose gel; a single line of precipitate is formed.
Relationships
The virus is distantly serologically related to tobacco ringspot virus (type and eucharis mottle strains; Fig. 7) but the two viruses do not cross-protect in plants and apparently do not form pseudo-recombinants. Moreover, the type and eucharis mottle strains of tobacco ringspot virus are not closely related to one another and could be considered to be distinct viruses (Fig. 7) (Salazar & Harrison, 1978b). In serological and plant-protection tests, the potato calico strain behaves very like the type strain of potato black ringspot virus (Fribourg, 1977; Salazar & Harrison, 1978b).
Potato black ringspot virus therefore belongs to the nepovirus group but no serological relationship was detected to the following other members: arabis mosaic, artichoke Italian latent, cacao necrosis, cherry leaf roll, grapevine fanleaf, myrobalan latent ringspot, raspberry ringspot, strawberry latent ringspot, tomato black ring and tomato ringspot viruses.
Stability in Sap
In Nicotiana clevelandii sap, the thermal inactivation point (10 min) is between 58 and 60°C, dilution end-point about 10-4 and infectivity is retained at 20°C for 9 to 10 days (Salazar & Harrison, 1977).
Purification
Extracts of inoculated and systemically infected leaves of N. clevelandii can be clarified by adding n-butanol to 8.5% (v/v) but larger virus yields are obtained when extracts are clarified with chloroform as described by Rezaian & Francki (1973). The following method (Salazar & Harrison, 1978a) is satisfactory. Triturate every 100 g tissue in 100 ml 0.06 M phosphate buffer, pH 7.0, containing 0.1% thioglycollic acid, together with 100 ml chloroform. Allow the suspension to stand for 30 min before centrifuging at 10,000 g for 20 min. To supernatant fluid add polyethylene glycol to 6% (w/v) and sodium chloride to 0.3 M, and stir for 1 h at 4°C. Centrifuge for 10 min at l0,000 g and suspend sediment overnight in 0.06 M phosphate buffer, pH 7.0, containing 0.01 M ethylenediamine-tetraacetate. Purify the virus further by two or more cycles of differential centrifugation, and by sucrose gradient centrifugation. The yield is 80-180 mg virus per kg leaf tissue.
Properties of Particles
Purified preparations contain three sedimenting components observed by analytical or density gradient centrifugation: empty protein shells without RNA (T) and two nucleoprotein components (M and B) containing 28 and 41% RNA respectively. M component particles contain RNA-2 and are not infective; B component particles contain either one molecule of RNA-1 or (probably) two molecules of RNA-2 and are very infective. Addition of M component particles to B component particles increases the infectivity only slightly (Salazar & Harrison, 1978a).
Sedimentation coefficient (s20,w) at infinite dilution (svedbergs): 49 (T), 84 (M) and 117 (B).
A260/A280: 0.63 (T), 1.78 (M), 1.97 (B).
Particle Structure
Particles are isometric and appear about 25 nm in diameter when stained with 2% phosphotungstate at pH 6.5 or with uranyl formate/NaOH. Particles have a somewhat angular outline (Fig. 6) and some are penetrated by the stains (Salazar & Harrison, 1978a).
Particle Composition
Nucleic acid: RNA, two species, probably single-stranded. M. Wt, estimated by polyacrylamide gel electrophoresis in non-denaturing conditions, are 2.5 x 106 (RNA-1) and 1.5 x 106 (RNA-2). Preparations of either species are poorly infective whereas mixtures are much more infective and both species probably are needed for infection (Salazar & Harrison, 1978a, 1978b).
Protein: A single polypeptide species with M. Wt c. 59,000 is found by polyacrylamide gel electrophoresis. A protease-sensitive structure needed for infection seems to be associated with the RNA (Salazar & Harrison, 1978a).
Relations with Cells and Tissues
Not studied.
Notes
Potato black ringspot virus can be distinguished from most other viruses reported from potato by its particle shape and size, together with its ability to infect Chenopodium amaranticolor, Cucumis sativus and Phaseolus vulgaris systemically. However, it cannot be distinguished in this way from tomato black ring virus, which occurs in potato in Western Europe, causing symptoms resembling those of potato black ringspot virus. Serological tests are needed to distinguish and identify these two viruses.
Figures

Systemic symptoms in graft-inoculated potato cv. Maris Bard (photograph: courtesy of the Scottish Horticultural Research Institute).

Lesions in inoculated leaf and systemic symptoms in Chenopodium amaranticolor (photograph: courtesy of the Scottish Horticultural Research Institute).

Systemic necrosis and distortion in Lycopersicon esculentum cv. Kondine Red (photograph: courtesy of the Scottish Horticultural Research Institute).

Systemic ringspotting in Nicotiana tabacum White Burley (photograph: courtesy of the Scottish Horticultural Research Institute).

Necrotic ringspots in inoculated Beta vulgaris leaf (photograph: courtesy of the Scottish Horticultural Research Institute).
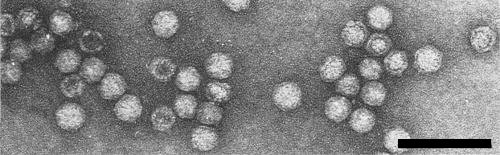
Virus particles stained with uranyl acetate. Bar represents 100 nm. (Photograph: courtesy of the Scottish Horticultural Research Institute.)

Gel-diffusion serological test using antiserum to the blueberry isolate of tobacco ringspot virus (well I). Peripheral wells contain the homologous virus (well B), potato black ringspot virus (well P) and eucharis mottle virus (well E). (Photograph: courtesy of the Scottish Horticultural Research Institute.)
References list for DPV: Potato black ringspot virus (206)
- Fribourg, Phytopathology 67: 174, 1977.
- Rezaian & Francki, Virology 56: 238, 1973.
- Salazar, Investnes agropec. Peru 3: 3, 1972.
- Salazar & Harrison, Nature, Lond. 265: 337, 1977.
- Salazar & Harrison, Ann. appl. Biol. 90: 375, 1978a.
- Salazar & Harrison, Ann. appl. Biol. 90: 387, 1978b.