Details of DPV and References
DPV NO: 209 July 1979
Family: Secoviridae
Genus: Comovirus
Species: Cowpea severe mosaic virus | Acronym: CPSMV
Cowpea severe mosaic virus
C. P. de Jager Department of Virology, Agricultural University, Wageningen, The Netherlands
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Probably first reported by
Smith (1924), later described by
Dale (1949) and
Shepherd (1964).
Synonyms
- Cowpea mosaic virus, severe strain (Rev. appl. Mycol. 43:
2773; Rev. Pl. Path. 53: 2381)
- Cowpea mosaic virus, severe subgroup (Rev. Pl. Path. 57: 4859)
- Arkansas cowpea mosaic virus (Rev. appl. Mycol. 43: 2772)
- Trinidad cowpea mosaic virus (Rev. appl. Mycol. 41: 564)
- Cowpea mosaic virus, severe subgroup (Rev. Pl. Path. 57: 4859)
-
A beetle-transmitted virus with isometric particles (diam. c. 25 nm), occurring in leguminous crops and weeds. Readily mechanically transmissible. The virus has two kinds of nucleoprotein particle with identical morphology but containing different RNA molecules, both of which are essential for infection. Occurs in the western hemisphere.
Main Diseases
Frequently found in mosaic-diseased cowpea (Vigna unguiculata). Incidence in this crop may be up to 100% (Dale, 1949; Van Hoof, 1963). About 50% reduction in fresh plant weight and in number and weight of pods is reported (Debrot & De Rojas, 1967). Also obtained from severely diseased soybeans (Thongmeearkom & Goodman, 1976; Thongmeearkom, Paschal & Goodman, 1978) and from other leguminous crops growing near infected cowpea plants (Dale, 1949). The common weed Phaseolus lathyroides is a major reservoir of the virus, and 80 to 100% of the plants can be infected (Alconero & Santiago, 1973; Lima & Nelson, 1977).
Geographical Distribution
Found in New World Countries: USA, Trinidad, Puerto Rico, El Salvador, Costa Rica, Venezuela, Surinam, Brazil and Peru.
Host Range and Symptomatology
Only leguminous plants are reported as natural hosts. Experimental host range includes many species of Leguminosae but relatively few hosts from other families. Almost all hosts develop necrotic or chlorotic lesions in inoculated leaves. Systemic symptoms are mottle or mosaic, often with severe blistering and distortion of leaflets. Some hosts show systemic necrosis and the plants may collapse. Cowpea and bean varieties differ in severity of symptoms, some bean varieties reacting with necrotic local lesions only (Agrawal, 1964; Caner, Silberschmidt & Flores, 1969; Debrot & De Rojas, 1967). In some Latin American locations, resistance was found in a few entries of the International Cowpea Disease Nursery program (Anon., 1974; Allen, 1977).
-
Diagnostic species
- Chenopodium amaranticolor.
Very small necrotic local lesions are produced in inoculated leaves. Plants are not invaded systemically. - Vigna unguiculata (cowpea) cv. Blackeye Early Ramshorn. Local symptoms in primary leaves are chlorotic lesions and often scattered red necrotic spots (Fig. 1, left). Parts of main veins turn red and become necrotic (Fig. 1, right). Trifoliolate leaves show severe mosaic with blistering, distortion and necrosis (Fig. 2). Plants inoculated as young seedlings show necrosis of the epicotyl just below the primary leaves (Fig. 3) and many then collapse. Isolates of the Arkansas type do not induce systemic necrosis; mosaic and distortion are less severe and lower trifoliolate leaves show pronounced vein yellowing.
-
Propagation species
- Vigna unguiculata
cv. Blackeye Early Ramshorn is a good source of virus for purification and for maintaining cultures.Assay species
- Chenopodium amaranticolor
and Phaseolus vulgaris cv. Pinto UI 114 may be used for local lesion assay. For local lesion transfer Pinto beans should preferably be used because the lesions contain much virus and little inhibitor.
Strains
The isolates from Arkansas and from Trinidad and Puerto Rico seem to differ in host range (Shepherd, 1964), or symptomatology (Thongmeearkom & Goodman, 1978a) and antigenic properties (Shepherd, 1963; Thongmeearkom & Goodman, 1978a; Pérez & Cortés-Monllor, 1971) and may therefore represent distinct strains. Wood (1972) isolated two nitrous acid mutants with changed local lesion type and different proportions of the three kinds of sedimenting particles.
Transmission by Vectors
Transmitted by leaf-feeding beetles, mainly of the family Chrysomelidae. About ten species are listed as vectors, of which Cerotoma ruficornis (= Andrector ruficornis) and C. trifurcata are among the most important (Smith, 1924; Dale, 1949, 1953; Walters & Barnett, 1964; Debrot & De Rojas, 1967; Pérez & Cortés-Monllor, 1970; Jansen & Staples, 1971; Slack & Fulton, 1971; Sanderlin, 1973; González, Moreno & Gámez, 1975). Beetles can acquire virus after only 5 min on the source plant (Dale, 1953) and may remain infective for 1 to 2 weeks (Dale, 1953; Walters & Barnett, 1964). Transmission efficiency and persistence in the vector increase with increasing length of the acquisition and inoculation access periods (Dale, 1953; Jansen & Staples, 1971) and decrease when soybean is used as source and test plant (Jansen & Staples, 1970). Dale (1953) found no latent period but Van Hoof (1963) established that the majority of infective beetles did not transmit virus to the first plant they fed on. Virus was recovered from regurgitated juice (Smith, 1924; Dale, 1953) and from haemolymph either after injection (Sanderlin, 1973) or natural uptake (Smith, 1924; Slack & Fulton, 1971). Beetles injected with virus transmitted it to test plants (Sanderlin, 1973).
Transmission through Seed
Transmission to 8% of seed was reported for the Trinidad isolate in asparagus bean (Vigna sesquipedalis) (Dale, 1949). The Arkansas isolate was transmitted to 10% of cowpea seed (Shepherd, 1964). Haque & Persad (1975) found 3.3 to 5.8% seed transmission in four out of seven cowpea selections in Trinidad.
Serology
The virus is strongly immunogenic. Rabbit antiserum titres of 1/8192 and 1/16,384 have been reported (Agrawal & Maat, 1964). Virus preparations tested by the Ouchterlony double diffusion method give a single line of precipitate, even though they show centrifugal and electrophoretic heterogeneity (Shepherd, 1963; Peréz & Cortés-Monllor, 1971; Thongmeearkom & Goodman, 1978a).
Relationships
Chant (1962) suggested a strain relationship between the cowpea mosaic virus isolated in Trinidad (Dale, 1949) and the cowpea yellow mosaic virus isolated in Nigeria (Chant, 1959). Agrawal (1964) classified the Trinidad cowpea mosaic virus and two isolates from Surinam (Vu and Vs) as the severe strain of cowpea mosaic virus, and the Nigerian cowpea yellow mosaic virus and another isolate (SB) from Surinam as the yellow strain of cowpea mosaic virus. In the first Description of cowpea mosaic virus (Van Kammen, 1971) both strains were included. However, Swaans & Van Kammen (1973) argued that they should be considered distinct viruses on grounds of differences in host range, symptomatology and antigenic properties, absence of homology in nucleotide sequence, and lack of complementation between heterologous nucleoprotein components. Separating the two viruses may also be justified by the non-coincidence of resistance to severe and yellow strains in the majority of cowpea lines and varieties tested (Agrawal, 1964; Anon., 1974; Allen, 1977; Beier, 1978). The revised Description of cowpea mosaic virus (Van Kammen & De Jager, 1978) dealt only with the yellow strain; the name cowpea mosaic virus was retained for this virus because the SB isolate of this strain had been accepted as the type culture of the cowpea mosaic virus group (Fenner, 1976). Moreover, biochemical and biophysical properties of cowpea mosaic virus had been mainly determined for isolates of the yellow strain. The name cowpea severe mosaic virus was chosen for the severe strain.
Isolates of cowpea severe mosaic virus from various countries show great similarity in biological properties, and close antigenic relationship, though often not complete identity (Agrawal & Maat, 1964; Shepherd, 1963, 1964; Pérez & Cortés-Monllor, 1971; Fulton & Scott, 1977; Thongmeearkom & Goodman, 1978a). Isolates are distantly related serologically to other viruses of the comovirus group. A summary of these relationships is given in the Description of the comovirus group (Bruening, 1978). In addition, a serological relationship between Trinidad cowpea severe mosaic virus and pea mild mosaic virus was reported (Clark, 1972).
Stability in Sap
In vitro properties depend considerably on the source and assay hosts and on the experimental conditions. Using cowpea primary leaves as a source and Pinto beans for assaying the virus the following values were found. Thermal inactivation point between 65 and 70°C; dilution end-point between 10-4 and 10-5; longevity in vitro, at a mean temperature of 23 to 24°C, 1 to 5 days (Agrawal, 1964; Debrot & De Rojas, 1967).
Purification
The virus can be purified by clarification with butanol and chloroform and precipitation with polyethylene glycol as described for cowpea mosaic virus (Van Kammen & De Jager, 1978).
Properties of Particles
Purified preparations contain three centrifugal components: top (T), consisting of empty protein shells; and two nucleoproteins, middle (M) and bottom (B), the particles of which are morphologically and serologically identical (Shepherd, 1964) but contain RNA molecules of different M. Wt (Thongmeearkom & Goodman, 1978a). T and B components are usually present in small amounts (Fig. 4). Separated M and B components (or the RNA species obtained from them) are non-infective but are infective when mixed. Infectivity is also restored when the M and B components are derived from different mutants of the virus (Wood, 1972) or when the separated RNA species are from different isolates (Thongmeearkom & Goodman, 1978a). No increase of infectivity was observed in heterologous mixtures of components of cowpea severe mosaic and cowpea mosaic viruses (Van Kammen, 1968).
Sedimentation coefficients, s°20,w (svedbergs): 58 (T); 95 (M); 115 (B).
A260/A280: 1.61 (unfractionated virus).
Buoyant density in CsCl (g/ml): density of M component changes from 1.386 to 1.404 when the pH is raised from 6.5 to 8.5. B component bands at two densities (1.403 and 1.443) at pH 6.5, but only at 1.443 at pH 8.5 (Wood, 1971).
The Puerto Rican isolate is reported to have only one electrophoretic component under a variety of conditions but other isolates contain two or more electrophoretic components (Agrawal, 1964; A. Van Kammen & C. P. De Jager, unpublished results). Upon treatment with trypsin the Puerto Rican virus is partially converted to a slower migrating form (Thongmeearkom & Goodman, 1978b).
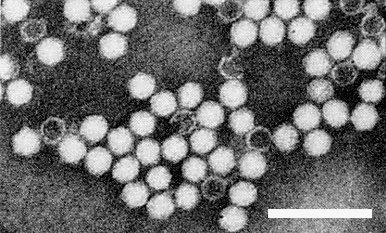
Particle Structure
Particles are isometric with a diameter of about 25 nm (Fig. 5).
Particle Composition
Nucleic acid: Purified virus yields two RNA species with different M. Wt, probably occurring separately in the M and B components. The RNA from the B component carries genetic determinants for local lesion type in Pinto bean and symptom type in cowpea. The smaller RNA determines antigenic specificity (Wood, 1972; Thongmeearkom & Goodman, 1978a).
Protein: Polyacrylamide gel electrophoresis of SDS-degraded virus shows the presence of three proteins, which, by analogy with cowpea mosaic virus, may be assumed to be a larger capsid protein and two M. Wt forms of a smaller capsid protein (Geelen, Van Kammen & Verduin, 1972; Thongmeearkom & Goodman, 1978b).
Relations with Cells and Tissues
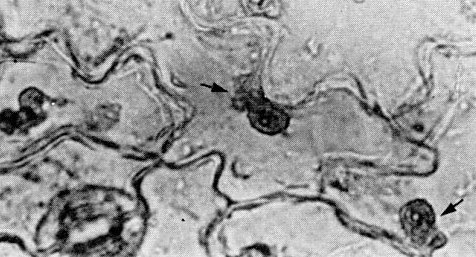
After staining epidermal strips of pea and cowpea with phloxine, amorphous inclusion bodies near or surrounding the nucleus can be seen in the light microscope (Fig. 6) (Agrawal, 1964; Swaans & Van Kammen, 1973). In serological precipitation tests the virus was found to reach higher concentrations in inoculated primary leaves than in non-inoculated trifoliolate leaves of cowpea (Pérez & Cortés-Monllor, 1971).
Notes
Cowpea severe mosaic virus may be distinguished from cowpea mosaic virus by its failure to infect Chenopodium amaranticolor systemically, its high M/B component ratio, and by its antigenic specificity. All these properties, together with particle morphology, distinguish cowpea severe mosaic virus from other viruses giving mosaic symptoms in Vigna spp. viz. cowpea aphid-borne mosaic virus (Bock & Conti, 1974); cowpea chlorotic mottle virus (Bancroft, 1971); cowpea mild mottle virus (Brunt & Kenten, 1974); sunnhemp mosaic virus (Kassanis & Varma, 1975); a strain of southern bean mosaic virus (Shepherd, 1971); a strain of bean common mosaic virus (Sachchidananda et al., 1973), and cowpea mottle virus (Shoyinka et al., 1978).
Figures

Symptoms in cowpea cv. Blackeye Early Ramshorn. Local symptoms in primary cowpea leaves, (left) chlorotic lesions and scattered necrotic spots, (right) chlorotic lesions and veinal necrosis. The virus is the Vs isolate from Surinam.

Symptoms in cowpea cv. Blackeye Early Ramshorn. Systemic symptoms in a severely diseased cowpea plant. The virus is the Vs isolate from Surinam.
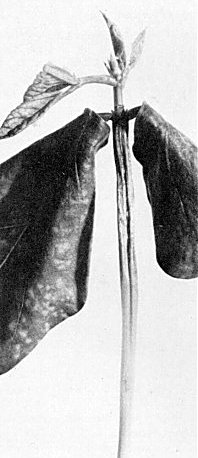
Symptoms in cowpea cv. Blackeye Early Ramshorn. Necrosis of the epicotyl after inoculation of primary leaves. The virus is the Vs isolate from Surinam.
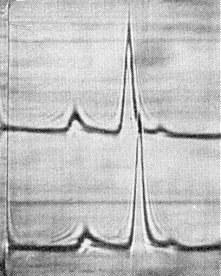
Schlieren patterns obtained by analytical centrifugation of virus purified from cowpea primary leaves 7 (upper line) and 9 (lower line) days after inoculation. Sedimentation is from left to right. The virus is the Vs isolate from Surinam.
References list for DPV: Cowpea severe mosaic virus (209)
- Agrawal, Meded. LandbHoogesch. Wageningen 64(5): 53 pp., 1964.
- Agrawal & Maat, Nature, Lond., 202: 674, 1964.
- Alconero & Santiago, Phytopathology 63: 120, 1973.
- Allen, Trop. Grain Legume Bull. 8: 28, 1977.
- Anon., Rep. int. Inst. trop. Agric., Ibadan, Nigeria, p. 108, 1974.
- Bancroft, CMI/AAB Descriptions of Plant Viruses 49, 4 pp., 1971.
- Beier, Abstr. 4th int. Congr. Virol: 262, 1978.
- Bock & Conti, CMI/AAB Descriptions of Plant Viruses 134, 4 pp.,1974.
- Bruening, CMI/AAB Descriptions of Plant Viruses 199, 5 pp., 1978.
- Brunt & Kenten, CMI/AAB Descriptions of Plant Viruses 140, 4pp., 1974.
- Caner, Silberschmidt & Flores, Biológico 35: 13, 1969.
- Chant, Ann. appl. Biol. 47: 565, 1959.
- Chant, Ann. appl. Biol. 50: 159, 1962.
- Clark, N. Z. Jl agric. Res. 15: 846, 1972.
- Dale, Ann. appl. Biol. 36: 327, 1949.
- Dale, Ann. appl. Biol. 40: 384, 1953.
- Debrot & De Rojas, Agronomía trop. 17: 3, 1967.
- Fenner, Intervirology 7: 1, 1976.
- Fulton & Scott, Fitopatol. Brasileira 2: 9, 1977.
- Geelen, Van Kammen & Verduin, Virology 49: 205, 1972.
- González, Moreno & Gámez, Proc. Am. Phytopath. Soc. 2: 75, 1975.
- Haque & Persad, in Tropical Diseases of Legumes, p. 119, ed. J.Bird & K. Maramorosch, New York: Academic Press, 1975.
- Jansen & Staples, Pl. Dis. Reptr 54: 1053, 1970.
- Jansen & Staples, J. econ. Ent. 64: 365, 1971.
- Kassanis & Varma, CMI/AAB Descriptions of Plant Viruses 153, 4pp., 1975.
- Lima & Nelson, Pl. Dis. Reptr 61: 864, 1977.
- Pérez & Cortés-Monllor, Pl. Dis. Reptr 54: 212, 1970.
- Perez & Cortés-Monllor, J. Agric. Univ. P. Rico 60: 184, 1971.
- Sachchidananda, Singh, Prakash & Verma, Z. PflKrankh. PflPath.PflSchutz 80: 88, 1973.
- Sanderlin, Phytopathology 63: 259, 1973.
- Shepherd, Phytopathology 53: 865, 1963.
- Shepherd, Phytopathology 54: 466, 1964.
- Shepherd, CMI/AAB Descriptions of Plant Viruses 57, 4 pp., 1971.
- Shoyinka, Bozarth, Reese & Rossel, Phytopathology 68: 693, 1978.
- Slack & Fulton, Virology 43: 728, 1971.
- Smith, Science, N.Y. 60: 268, 1924.
- Swaans & Van Kammen, Neth. J. Pl. Path. 79: 257, 1973.
- Thongmeearkom & Goodman, Proc. Am. Phytopath. Soc. 3: 209, 1976.
- Thongmeearkom & Goodman, Virology 85: 75, 1978a.
- Thongmeearkom & Goodman, J. gen. Virol. 41: 155, 1978b.
- Thongmeearkom, Paschal & Goodman, Phytopathology 68: 1549, 1978.
- Van Hoof, Surin. Landb. 11: 131, 1963.
- Van Kammen, Virology 34: 312, 1968.
- Van Kammen, CMI/AAB Descriptions of Plant Viruses 47, 4 pp.,1971.
- Van Kammen & De Jager, CMI/AAB Descriptions of Plant Viruses 197, 6 pp., 1978.
- Walters & Barnett, Phytopathology 54: 911, 1964.
- Wood, Virology 43: 511, 1971.
- Wood, Virology 49: 592, 1972.