Details of DPV and References
DPV NO: 210 July 1979
Family: Geminiviridae
Genus: Curtovirus
Species: Beet curly top virus | Acronym: BCTV
Beet curly top virus
P. E. Thomas United States Department of Agriculture, Science and Education Administration
G. I. Mink Washington State University, Irrigated Agriculture Research and Extension Center, Prosser, Washington 99350, USA
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by Ball (1909).
- Selected synonyms:
- Sugar beet curly top virus (Boncquet & Hartung, 1915)
- Sugar beet curly-leaf (virus) (Ball, 1909)
- Western yellow blight virus (Rev. appl. Mycol. 7: 282)
- Tomato yellows (virus) (Rev. appl. Mycol. 7: 478)
- Sugar beet curly-leaf (virus) (Ball, 1909)
- A virus with isometric particles about 20 nm in diameter occurring singly or in pairs (geminate). It has a wide host range, and is transmitted by two species of leafhopper, Circulifer tenellus and C. opacipennis, in which it circulates without multiplying. The virus is restricted to the phloem and is transmissible mechanically only by special procedures. Of great historical and economic importance in Western USA.
Main Diseases
Causes debilitating, often lethal, yellows-type diseases, usually accompanied by leaf-curling and distortion, in red beet and sugar beet, Swiss chard, spinach, tomato, pepper, bean, cucurbits, flax, and in many ornamental and uncultivated species. Causes a minor disease of potato called green dwarf (Giddings, 1954).
Geographical Distribution
Occurs in the arid and semi-arid regions of Western North America from Mexico to Canada, and in the Eastern Mediterranean Basin, from where it appears to have originated (Bennett & Tanrisever, 1958). The vectors are confined to arid and semi-arid climates.
Host Range and Symptomatology
The virus is not transmissible by rub-inoculation methods but is transmissible infrequently by pin-pricking (Severin, 1924) and by injection under pressure (Mumford, 1972). Transmission by means of leafhoppers occurs to a very wide range of hosts, including more than 300 species in 44 families of dicotyledonous plants (Bennett, 1971), especially in the Chenopodiaceae, Compositae, Cruciferae, Leguminosae and Solanaceae. No monocotyledonous hosts are known. Many species are susceptible to specific virus strains and immune to others (Freitag & Severin, 1936). Many hosts to which the vector transmits the virus will not sustain the vector (Thomas, 1977). Vein-clearing and some degree of vein-swelling and distortion in the youngest leaves is the earliest and most universal symptom, occurring in nearly all hosts, including those that are tolerant or exhibit ‘recovery’. Other symptoms include rapid collapse and death; rigidity, dwarfing and yellowing of plants; curling, twisting and distortion of young leaves and growing points; growth of axillary buds; phloem necrosis; and exudation of fluid from the phloem. Several hosts develop no symptoms (Freitag & Severin, 1936; Thomas, 1969).
- Diagnostic species
- Beta vulgaris
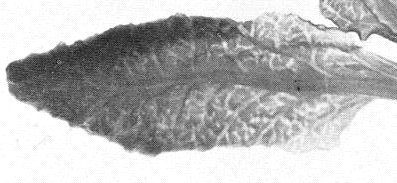
(sugar beet). Vein-clearing in young leaves (Fig. 2) is followed by upward and inward leaf-rolling. A conspicuous vein-swelling and galling (Fig. 1) produces a roughened lower leaf surface, rasp-like to the touch. Droplets of phloem exudate often form (Fig. 4) on petioles and large veins. Phloem necrosis may be observed as dark concentric rings in transverse sections of roots. - Cucumis sativus (cucumber). Seedlings are killed. New growth on older
plants is dwarfed, internodes are shortened, the margins of leaf blades roll
upward, and the rosetted growing points face upward. Fruits are crooked or
otherwise malformed.
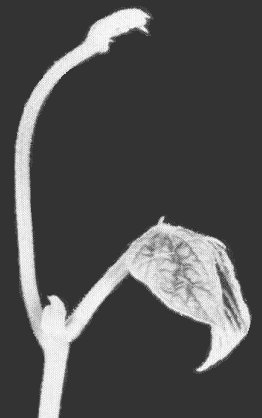
- Lycopersicon esculentum (tomato). Young opposite leaflets twist, tend to roll upward toward one another, and sometimes cross over the top of the petiole (Fig. 5, Fig. 7). Leaves become thickened, crisp, and yellowed with purplish veins. Plants take on a rigid, upright appearance (Fig. 6). All fruits turn red prematurely, and the plants decline and die.
- Nicotiana tabacum (tobacco). Young leaves are dwarfed with swollen, distorted veins and downward-rolled margins, forming a rosette at the growing point. Normal growth resumes after a few weeks. ‘Recovery’ occurs in all Nicotiana species (Bennett, 1971).
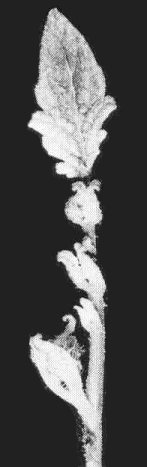
- Phaseolus vulgaris (French bean) cv. Bountiful. A sharp epinastic bend occurs at the base of the first trifoliolate leaf (Fig. 8) in plants inoculated in the cotyledon stage. Plants may die immediately or axillary buds may proliferate (Fig. 9) producing brittle, curled leaflets showing vein-clearing and vein-swelling, and crinkled upper surfaces.
- Lycopersicon esculentum (tomato). Young opposite leaflets twist, tend to roll upward toward one another, and sometimes cross over the top of the petiole (Fig. 5, Fig. 7). Leaves become thickened, crisp, and yellowed with purplish veins. Plants take on a rigid, upright appearance (Fig. 6). All fruits turn red prematurely, and the plants decline and die.
- Propagation species
- Beta vulgaris
(sugar beet) is the preferred host for maintaining cultures; it is a good host for the vector and the roots may be stored refrigerated for several months. Phaseolus vulgaris cvs Romano and Bountiful (Mink & Thomas, 1974) and Nicotiana tabacum cv. Turkish (Mumford, 1974) are good sources for virus purification.- Assay species
- There are no known local-lesion hosts. Seedlings of Beta vulgaris (sugar beet) are used to assay infectivity of vectors that have fed on test plants or solutions.
Strains
Many genetically stable strains differing in virulence, symptomatology and host range have been described by Giddings (1938, 1944, 1954), Bennett (1963, 1971), Thomas (1970) and Mumford & Peay (1970). Some major variants are:
Strain 7 of Giddings (1944). Very low virulence, infecting only the most susceptible sugar beet cultivars.
Strain 11 of Giddings (1954). The most virulent strain of its day on Beta vulgaris.
Strain 66-10 of Mumford & Peay (1970). Typical of the strains of increased virulence on Beta vulgaris evolving in Western USA in the 1960s and 1970s.
Strain 31A of Thomas (1970). Extremely virulent in solanaceous plants but has low virulence in Beta vulgaris.
Strain Murale of Bennett (1971). The only American strain that causes symptoms in Chenopodium murale.
Transmission by Vectors
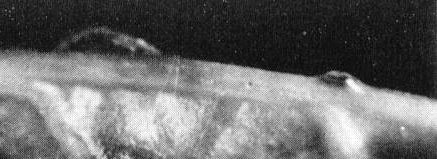
The leafhopper Circulifer tenellus (Fig. 3) is the only natural
vector known to occur in North America (Stahl & Carsner, 1923). C.
tenellus and C. opacipennis occur and transmit the virus in the
Mediterranean Basin (Kheyri & Alimoradi, 1969).
Virus-vector relations have been studied extensively only in North America.
The virus circulates but does not multiply in the vector and is not transmitted
through the egg. Leafhoppers transmit occasionally after an acquisition time
of 1 min (Severin, 1931) but frequency of transmission is greatest after
acquisition access periods of 2-3 days (Bennett & Wallace, 1938). The
minimum latent period in the vector is rarely less than 4 h (Severin, 1923).
The minimum probing period required for transmission is about 1 min (Bennett
& Wallace, 1938). Frequency of transmission depends on the amount of virus
in the vector which in turn depends on the virus content of the source plant
(Bennett, 1962). Highly infective vectors transmit the virus for life but the
proportion of test plants infected decreases when the vectors are maintained
on virus-immune plants or on a series of healthy susceptible plants (Freitag,
1936). Ability to transmit may be regained by renewed feeding on a diseased
plant or virus preparation (Bennett & Wallace, 1938).
Transmission frequency is high both to hosts and to non-hosts of the vector, particularly during the first few minutes of probing (Thomas, 1977).
Transmission through Seed
The virus is not seed-transmitted in beet (Bennett & Esau, 1936); although virus accumulates in the perisperm as the seed matures, it does not invade the embryo.
Transmission by Dodder
At least three species of Cuscuta can transmit at relative efficiencies dependent upon the host species (Bennett, 1944). Dodder itself is not infected, although it may contain relatively high concentrations of virus and show phloem necrosis when growing on an infected plant. Dodder transmission can be used to recover the virus from plants that are not good hosts of the vector.
Serology
The virus is strongly immunogenic but occurs in low concentrations in plants. Antiserum has been produced in rabbits by intramuscular injection. The latex flocculation serological assay is 100 times more sensitive than the agar double diffusion assay and only four times less sensitive than transmission to plants by leafhoppers (Mumford, 1977). Enzyme-linked immunosorbent assay is as sensitive for detecting virus in single leafhoppers as testing their infectivity on plants (Mumford & Thornley, 1978).
Relationships
North American strains are recognized on the basis of virulence, symptomatology
and host range. Serological relationships have not been determined. There is
no appreciable interference or cross protection among these strains either in
plant hosts or in vector insects. Evidence indicates that the North American
virus was introduced with its vector from the Mediterranean region, perhaps
with early settlers, and reciprocal transmission tests with virus isolates and
vectors suggest that the viruses in the two regions are essentially the same
(Bennett, 1971).
Four yellows-type diseases caused by leafhopper-transmitted agents - Argentine beet curly top (Bennett et al., 1946), Brazilian beet curly top (Bennett & Costa, 1949), Brazilian tomato curly top (Costa, 1952) and summer death of bean in Australia (Ballantyne, 1970) - have similarities with beet curly top which suggest affinities between the causal viruses. None are transmitted by C. tenellus, but similarities exist in symptomatology, host range, transmission requirements, vector-virus relations and simple virus properties. Furthermore, sugar beet cultivars developed for resistance to the North American virus ranked in the same order for resistance to the Argentinian, Brazilian and North American viruses. Similarly, bean cultivars selected for resistance to the North American virus were resistant to bean summer death virus in Australia. Serological relationships between these viruses have not been studied.
The fact that beet curly top virus has paired (geminate) particles possibly containing DNA suggests that beet curly top virus may ultimately be included in the geminivirus group.
Stability in Sap
Stability was determined by measuring the ability of the vector to transmit after feeding artificially on virus-containing extracts (Severin & Freitag, 1933; Bennett, 1935). The thermal inactivation point (10 min) is about 80°C and the dilution end-point about 1/1000 in sugar beet root juice. Dilution end-point is about 1/24,000 in extracts of viruliferous vectors. Infectivity is retained for up to 8 days at room temperature and more than 11 months at -18°C in sugar beet root juice; and for up to 4 months in dried sugar beet tissue in the open air and more than 8 years over calcium chloride. Infectivity in phloem exudate is retained after exposure to pH 9.1 for 2 h, but is lost after a similar exposure at pH 2.9 or lower. Infectivity in extracts is readily carried out of solution by almost any type of precipitate, including those formed as a result of heating or adding alcohol to plant extracts (Bennett, 1935).
Purification
1. (Bennett, 1935). Very small amounts of relatively pure virus may be obtained by resuspending precipitates produced by mixing equal volumes of phloem exudate and 95% ethanol. The precipitate is washed in a volume of 50% ethanol equal to the original volume, dried, and resuspended in water or 0.001 M phosphate buffer (pH 7.0).
2. (Mumford, 1974). Homogenize infected tobacco leaf in 0.01 M phosphate buffer (pH 7.0) containing 0.01 M Na2SO3 and 0.001 M ethylenediamine-tetraacetate (EDTA) (2 ml/g tissue), strain, centrifuge at low speed, and emulsify the supernatant fluid in one-half its volume of chloroform + butanol (1:1 v/v). Break the emulsion by low speed centrifugation, bring aqueous phase to 10% (w/v) polyethylene glycol, M. Wt 6000 (PEG) and to 1% (w/v) NaCl and centrifuge at low speed to precipitate the virus. Resuspend the pellet in 0.001 M phosphate buffer containing 0.001 M EDTA (10 ml/100g original tissue), hold overnight at 5°C, centrifuge at low speed, saving the supernatant fluid, and extract the pellet three times with 0.001 M phosphate containing 0.001 M EDTA. Concentrate virus in the combined supernatant fluids by precipitation with PEG + NaCl to 2 ml/100 g original tissue. Centrifuge on 10-40% sucrose gradients in 0.001 M phosphate buffer containing 0.001 M EDTA, combine infective zones, concentrate by ultrafiltration, and chromatograph through a column of agarose beads, eluting with 0.001 M phosphate buffer (pH 7.0).
3. (Mink & Thomas, 1974). Use stem and petiole tissue of Phaseolus vulgaris cv. Bountiful inoculated at the crook-neck stage and harvested at the first trifoliolate leaf stage. Disrupt tissue in eight volumes distilled water, strain through cheesecloth, add 12 g activated charcoal per 100 g original tissue, centrifuge at low speed, and concentrate virus in the supernatant fluid by two cycles of differential ultracentrifugation (39,000 rev/min for 1 h and 10,000 rev/min for 10 min). Centrifuge on rate zonal sucrose (10-40%) gradients and recover the infective zone.
Properties of Particles
Sedimentation coefficient (s20,w): about 82 S
estimated for single particles in sucrose gradients (Mink & Thomas, 1974).
Two components, of about 55 S and 86 S were reported by Egbert,
Egbert & Mumford (1976).
A260/A280: 1.68-1.70.
Particle Structure
Purified preparations contain small isometric particles 18-22 nm in diameter (Mumford, 1974) occurring both singly and paired (‘geminate particles’; Fig. 11, Fig. 12). Single and paired particles are both infective (Egbert et al., 1976).
Particle Composition
Nucleic acid: The type of nucleic acid has not been determined unequivocally. Purified preparations show a positive diphenylamine test and sensitivity to DNase suggesting that DNA is present (Magyarosy et al., 1977).
Protein: No information.
Relations with Cells and Tissues
Restriction of curly top virus to, and transport in, the phloem was proved in classical experiments (Bennett, 1937; Esau, 1935). Single and paired particles were found in the nuclei of phloem parenchyma cells by electron microscopy but not in the cytoplasm of such cells nor in sieve elements or companion cells (Esau, 1977). The particles did not appear to have any relation to the nucleoli, and sometimes appeared as ribbon-like strands of paired particles (Fig. 10). Fluorescent antibody techniques detected virus particle antigen in the nuclei early in infection and later throughout the cytoplasm of phloem parenchyma cells (Mumford & Thornley, 1977). Antigen was also detected in a few cortical cells adjacent to the cambium.
Notes
In the geographical regions where beet curly top virus is present, no other viruses are known that produce the definitive symptoms of curly top disease on sugar beet or have the same vector.
Figures
References list for DPV: Beet curly top virus (210)
- Ball, Bull. U.S. Dept. Agric. Bur. Entomol. 66: 33, 1909.
- Ballantyne, Pl. Dis. Reptr 54: 903, 1970.
- Bennett, J. agric. Res. 50: 211, 1935.
- Bennett, J. agric. Res. 54: 479, 1937.
- Bennett, Phytopathology 34: 905, 1944.
- Bennett, Phytopathology 52: 538, 1962.
- Bennett, J. Am. Soc. Sug. Beet Technol. 2: 515, 1963.
- Bennett, Monogr. Am. Phytopath. Soc. No. 7, 81 pp., 1971.
- Bennett & Costa, J. agric. Res. 78: 675, 1949.
- Bennett & Esau, J. agric. Res. 53: 595, 1936.
- Bennett & Tanrisever, J. Am. Soc. Sug. Beet Technol. 10: 189, 1958.
- Bennett & Wallace, J. agric. Res. 56: 31, 1938.
- Bennett, Carsner, Coons & Brandes, J. agric. Res. 72: 19, 1946.
- Boncquet & Hartung, Phytopathology 5: 348, 1915.
- Costa, Phytopathology 42: 396, 1952.
- Egbert, Egbert & Mumford, Abs. Ann. Meet. Am. Soc. Microbiol. 1976, p. 258, 1976.
- Esau, Am. J. Bot. 22: 149, 1935.
- Esau, J. Ultrastruct. Res. 61: 78, 1977.
- Freitag, Hilgardia 10: 305, 1936.
- Freitag & Severin, Hilgardia 10: 263, 1936.
- Giddings, J. agric. Res. 56: 883, 1938.
- Giddings, J. agric. Res. 69:149, 1944.
- Giddings, Phytopathology 44: 125, 1954.
- Kheyri & Alimoradi, Report of the Sugarbeet Seed Institute, Karaj Entomol. Res. Div., Tehran, Iran, 54 pp., 1969.
- Magyarosy, Schürmann, Buchanan & Finlay, Proc. Am. Phytopath. Soc. 4:161, 1977.
- Mink & Thomas, Phytopathology 64: 140, 1974.
- Mumford, Phytopathology 62:1217, 1972.
- Mumford, Phytopathology 64:136, 1974.
- Mumford, Phytopathology 67: 949, 1977.
- Mumford & Peay, J. Am. Soc. Sug. Beet Technol. 16: 185, 1970.
- Mumford & Thornley, Phytopathology 67: 1313, 1977.
- Mumford & Thornley, Phytopathology News 12:192, 1978.
- Severin, Rep. Calif. Agric. Exp. Stn 1922-23: 127, 1923.
- Severin, Phytopathology 14: 80, 1924.
- Severin, Hilgardia 6: 253, 1931.
- Severin & Freitag, Hilgardia 8:1, 1933.
- Stahl & Carsner, J. econ. Ent. 16: 476, 1923.
- Thomas, Pl. Dis. Reptr 53: 548, 1969.
- Thomas, Phytopathology 60: 844, 1970.
- Thomas, Phytopathology 67: 903, 1977.