Details of DPV and References
DPV NO: 225 September 1980
Family: Secoviridae
Genus: Nepovirus
Species: Lucerne Australian latent virus | Acronym: LALV
Lucerne Australian latent virus
A. T. Jones Scottish Horticultural Research Institute, Invergowrie, Dundee, Scotland
R. L. S. Forster Scottish Horticultural Research Institute, Invergowrie, Dundee, Scotland
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
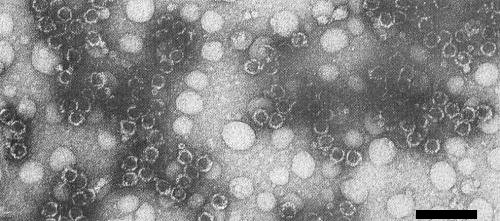
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by Taylor & Smith (1971), Blackstock
(1978) and Jones, Forster & Mohamed (1979).
- Synonym
- Lucerne latent virus (Rev. Pl. Path. 68, 5559)
- A virus with isometric particles c. 25 nm in diameter sedimenting as three components and containing two RNA species. It is sap-transmissible to several herbaceous hosts and is seed-transmitted in lucerne and Chenopodium quinoa. Reported to occur commonly in lucerne crops in Australia and New Zealand.
Main Diseases
Occurs symptomlessly in lucerne
(Medicago sativa) (Blackstock, 1978; Jones
et al. 1979).
Geographical Distribution
Reported only from lucerne in Australia and New Zealand (Jones et al., 1979).
Host Range and Symptomatology
Lucerne, whether naturally or experimentally infected, shows no foliar symptoms and is difficult to infect by mechanical inoculation (Blackstock, 1978; Jones et al., 1979). Several other leguminous species can be infected experimentally, including Lupinus angustifolius, Medicago spp., Phaseolus spp., Pisum sativum and some Trifolium spp. Most are infected symptomlessly (Blackstock, 1978; Jones et al., 1979). Other experimental hosts include members of the Chenopodiaceae and Solanaceae, but only a few of these produce symptoms.
- Diagnostic species
- Chenopodium amaranticolor. Systemic mottle
after 7-10 days.
- C. murale. Partially purified preparations induce chlorotic or necrotic local rings after 6-7 days (Fig. 1) followed occasionally by systemic necrotic spots.
- C. quinoa. Large necrotic local lesions after 5-7 days followed by systemic mottle or tip necrosis in 6-10 days (Fig. 3).
- Gomphrena globosa. Occasional systemic mottle in 8-17 days sometimes accompanied by epinasty or veinal yellowing (Fig. 2).
- Pisum sativum (pea). Inoculated leaves become yellow and collapse after 4-7 days. Symptomless systemic infection.
- C. murale. Partially purified preparations induce chlorotic or necrotic local rings after 6-7 days (Fig. 1) followed occasionally by systemic necrotic spots.
- Propagation species
- Chenopodium quinoa and Pisum sativum have been used (Blackstock, 1978; Jones et al., 1979). Gomphrena globosa is useful for maintaining cultures.
- Assay species
- The virus may be assayed by recording the proportion of C. quinoa plants that become systemically infected. For partially purified preparations, Chenopodium murale is a satisfactory local lesion host (Jones et al., 1979).
Strains
R. L. S. Forster (unpublished data) distinguished three New Zealand isolates by differences in host range and symptomatology. A New Zealand and an Australian isolate (TN) were indistinguishable serologically but differ in reported host ranges (Jones et al., 1979). Some of these apparent host range differences may be caused by differences in climate, concentration of virus inoculum and inoculation technique. Only two isolates have been identified serologically. The SM virus isolate reported from lucerne in Australia and originally considered to be a strain of lucerne Australian latent virus (Blackstock, 1978) is serologically unrelated to lucerne Australian latent virus (A. T. Jones, unpublished data).
Transmission by Vectors
No vector reported. The aphids Acyrthosiphon solani, Aphis craccivora and Myzus persicae did not transmit the virus from lucerne to lucerne. Limited tests gave no evidence of soil transmission (Blackstock, 1978). However, natural spread in lucerne appears to occur readily in some crops in Australia (Blackstock, 1978) and New Zealand (Jones et al., 1979; Ashby et al., 1979).
Transmission through Seed
Transmitted through seed of lucerne, Chenopodium amaranticolor and C. quinoa (Taylor & Smith, 1971; Blackstock, 1978; Jones et al., 1979). Virus was detected in pollen of Chenopodium quinoa, and pollen transmission of the virus to seed and progeny seedlings was detected (Blackstock, 1978). The virus was detected in the embryos of lucerne seed but not in the endosperm or testa (Blackstock, 1978).
Serology
The virus is weakly immunogenic giving an antiserum with a titre of 1/128-1/256. In gel double diffusion tests purified virus produces a single line of precipitate but sap of infected lucerne and other herbaceous hosts usually contains too little virus to react (Jones et al., 1979).
Relationships
In particle morphology, sedimentation pattern, host range and symptomatology, and in the number and M. Wt of its coat protein and RNA components, it resembles nepoviruses and should probably be placed in this group although no nematode vector is known. Purified virus preparations failed to react with antisera to 10 nepoviruses or to 18 other distinct plant viruses with isometric particles (Jones et al., 1979).
Stability in Sap
In Chenopodium quinoa sap a New Zealand isolate lost infectivity after dilution to 10-4, heating for 10 min at 55°C or storage for 4 days at 4°C. Sap diluted 1:2 in 0.04 M phosphate buffer, pH 7, was infective after storage for 8 days at 20°C (Jones et al., 1979). Australian isolates behaved similarly.
Purification
Purification of the virus is difficult because of the low concentration of particles in sap and the problem of removing contaminating host plant components. However, a New Zealand isolate was purified from systemically infected C. quinoa or Pisum sativum plants by extracting sap in 0.1 M borate buffer, pH 7.0, containing 0.2% 2-mercaptoethanol and clarifying with 15% (w/v) bentonite suspension. Further clarification and concentration was achieved by differential centrifugation and sucrose density gradient centrifugation. Virus yield from 100 g of infected pea plants was about 1-2 mg (Jones et al., 1979).
Properties of Particles
Purified preparations contain three sedimenting components designated top (T), middle (M) and bottom (B). T component is believed to be nucleic acid-free protein shells (Jones et al., 1979).
Sedimentation coefficient (s°20,w): 56 S (T), 128 S (M) and 133 S (B).
A260/A280: 1.71 (M + B).
Particle Structure
Particles stained well in most negative stains and a proportion of them were penetrated by the stain (Fig. 4, Fig. 5). Particles are isometric, about 24-25 nm in diameter (Jones et al., 1979).
Particle Composition
Nucleic acid: RNA, probably single-stranded (Jones et al., 1979). In polyacrylamide gels under non-denaturing conditions, RNA preparations from mixtures of M and B components contained two RNA species of estimated M. Wt 2.1 x 106 (RNA-2) and 2.4 x 106 (RNA-1). In sucrose density gradients the two RNA species had sedimentation coefficients of 32.5 S and 36 S corresponding to M. Wt of 2.1 x 106 and 2.6 x 106 respectively. RNA-1 and RNA-2 probably correspond to the nucleic acid species from B and M components respectively (Jones et al., 1979).
Protein: In polyacrylamide gels, protein preparations from components T and M + B contained a single polypeptide of estimated M. Wt 55,000 ± 1000 (Jones et al., 1979).
Relations with Cells and Tissues
No information.
Notes
The virus differs from other sap-transmissible viruses with isometric particles reported from lucerne such as cucumber mosaic, lucerne transient streak, pea enation mosaic and tobacco streak, and from alfalfa mosaic virus, in having different sedimentation profiles, and different protein M. Wt and RNA M. Wt; it is serologically unrelated to all these viruses and to the SM virus isolate from lucerne in Australia, originally considered to be a strain of lucerne Australian latent virus (Blackstock, 1978; Jones et al., 1979; A. T. Jones, unpublished data).
Figures

Necrotic local lesions in a leaf of Chenopodium murale (photograph courtesy Plant Diseases Division, DSIR, Auckland, New Zealand).
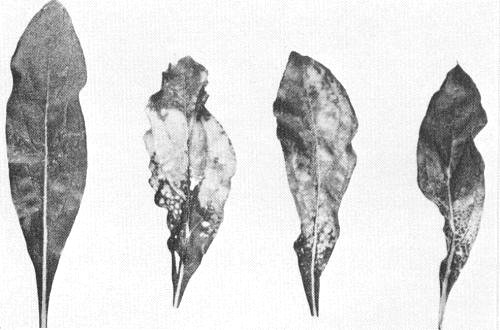
Range of symptoms induced in systemically infected leaves of Gomphrena globosa (photograph courtesy Plant Diseases Division, DSIR, Auckland, New Zealand).

Systemic tip death in C. quinoa (photograph courtesy Plant Diseases Division, DSIR, Auckland, New Zealand).
References list for DPV: Lucerne Australian latent virus (225)
- Ashby, Forster, Fletcher & Teh, N.Z. J. agric. Res. 22: 637, 1979.
- Blackstock, Aust. J. agric. Res. 19: 291, 1978.
- Jones, Forster & Mohamed, Ann. appl. Biol. 92: 49, 1979.
- Taylor & Smith, Rep. Victorian Pl. Res. Inst. No. 5: 20, 1971.