Details of DPV and References
DPV NO: 229 September 1980
Family: Bromoviridae
Genus: Alfamovirus
Species: Alfalfa mosaic virus | Acronym: AMV
This is a revised version of DPV 46
Alfalfa mosaic virus
E. M. J. Jaspars Department of Biochemistry, State University, Leiden, Netherlands
L. Bos Institute for Phytopathological Research, Wageningen, Netherlands
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by Weimer (1931, 1934) and Bancroft & Kaesberg (1958, 1960).
Selected synonyms
- Lucerne mosaic virus (Rev. appl. Mycol. 24: 513)
- Alfalfa virus 1 and 2 (Rev. appl. Mycol. 13: 488)
- Marmor medicaginis (Rev. appl. Mycol. 28: 514)
- Alfalfa virus 1 and 2 (Rev. appl. Mycol. 13: 488)
A virus with bacilliform particles of different lengths, the largest usually c. 60 nm, in which four species of single-stranded RNA of messenger polarity are separately packaged. The three largest RNA species comprise the genome; the fourth is a sub-genomic messenger for the coat protein. The three genome RNA species and either the fourth RNA or the coat protein are needed for infectivity. The virus is readily sap-transmissible, is seed-transmissible in some hosts and is transmitted in the non-persistent manner by aphids to a very wide range of host plants. Common in most countries.
Main Diseases
Causes various mosaics, mottles and malformations in lucerne (alfalfa; Medicago sativa) (Fig. 1) but is often symptomless in this host, especially during summer, and is most prevalent in old crops. Causes calico and tuber necrosis in potato, various symptoms in tobacco and garden lupin, mosaic in Malva parviflora and Viburnum opulus, yellow fleck in Caryopteris incana, white mottle in Philadelphus sp. and is one of the causes of mosaic in red and white clover, celery, celeriac and lettuce, of yellow mosaic in bean (Phaseolus vulgaris), cowpea (Vigna unguiculata), mung bean (V. radiata) and chilli pepper (Capsicum annuum) (Fig. 2), of necrosis and stunting in pea (Pisum sativum), of severe necrosis in tomato, and of wilting in chickpea (Cicer arietinum). It occurs naturally in many wild and cultivated species. In lucerne and clovers, crop yield is often reduced and predisposition to drought and winter injury increased.
Geographical Distribution
World-wide.
Host Range and Symptomatology
Occurs naturally and often symptomlessly in many herbaceous and some woody hosts (150 species in 22 families: Schmelzer, Schmidt & Beczner, 1973). It is transmissible to over 430 species of 51 dicotyledonous families (Hull, 1969; Crill, Hagedorn & Hanson, 1970; Schmelzer et al., 1973). Sap transmission is easy between most hosts, but may be difficult between some e.g. from lucerne to Phaseolus beans during summer. Symptoms depend greatly on virus strain, host variety and stage of growth, and environmental conditions. Infection may be latent or masked and recovery often occurs. In many species mottle or mosaic is bright yellow (calico); local and systemic necrosis may also occur.
-
Diagnostic species
- Chenopodium amaranticolor
and C. quinoa. Chlorotic or necrotic local lesions; the characteristic systemic chlorotic and necrotic flecking distinguishes alfalfa mosaic virus from cucumber mosaic virus. - Nicotiana tabacum (tobacco). Necrotic or chlorotic local lesions
(Fig. 4). Some strains
give no local reaction. Systemic symptoms usually are mild mottle, bright chlorotic vein banding,
coalescing ringspots
(Fig. 5) and, rarely, deformation
(Fig. 6). Plants commonly recover
(Fig. 5).
Enations are induced by some strains.
- Ocimum basilicum (basil). Systemic yellow mosaic.
- Phaseolus vulgaris (French bean). Many strains of the virus give necrotic local lesions (Fig. 3), sometimes coalescing, which appear in 3 to 5 days in most cultivars; other strains produce chlorotic local lesions or none at all but give systemic mild mottle, vein necrosis and leaf distortion.
- Pisum sativum (pea). In most cultivars local lesions and/or wilting of inoculated leaves with stem necrosis and plant death.
- Vicia faba (broad bean). Most strains give black necrotic local lesions, sometimes followed by a mild mottle, but more often by stem necrosis and plant death.
- Vigna unguiculata (cowpea). The commonest strains produce necrotic local lesions and do not infect systemically; others do not induce local lesions but produce various systemic symptoms.
- Ocimum basilicum (basil). Systemic yellow mosaic.
-
Propagation species
- Nicotiana tabacum
and N. glutinosa are suitable for maintaining cultures. N. tabacum cultivars, especially those hypersensitive to tobacco mosaic virus (e.g. ‘Samsun NN’), are good sources of virus for purification. It should be noted that virus concentration in tobacco soon reaches a high peak but rapidly declines thereafter to a very low level, and this loss of virus coincides with recovery from symptoms (Ross, 1941). Peak virus concentration and the time needed to reach it are influenced by inoculum dose (Fig. 8) (Verhoyen, 1966).Assay species
- Phaseolus vulgaris
and Vigna unguiculata are good assay hosts for strains that produce local lesions in these plants. Chenopodium amaranticolor and C. quinoa are also suitable.
Strains
Numerous strains or variants with minor differences have been described. Distinction is mainly by differential reaction on one or two selected hosts (notably Phaseolus vulgaris and Vigna unguiculata) and by differences in such properties as particle aggregation forms in tobacco cytoplasm, pollen and seed transmission and physico-chemical properties. Most molecular biological research has been done with strain AMV-S originating from lucerne (Gibbs & Tinsley, 1961), strain AMV 425 isolated from clover (Hagedorn & Hanson, 1963), the alfalfa yellow spot mosaic strain of Zaumeyer (1963) and strains AMV 15/64 and VRU of Hull (1969, 1970b). Symptomatologically different strains do not necessarily differ in amino acid composition and serological behaviour (Tremaine & Stace-Smith, 1969). A grouping of strains on the basis of the chemical properties of the coat proteins has been proposed by Kraal (1975).
Transmission by Vectors
Transmitted in the non-persistent manner by at least 14 aphid species (Crill et al., 1970). There is no latent period in the aphid and frequency of transmission is increased by starving the aphids before acquisition of virus (Swenson, 1952). Myzus persicae can acquire the virus from purified preparations through Parafilm membranes (Pirone, 1964).
Transmission through Seed
Presumably common in lucerne (Frosheiser, 1970; Hemmati & McLean, 1977); reported up to c. 10% in commercial seed (Beczner & Manninger, 1975; Hemmati & McLean, 1977) and up to c. 50% in seeds from individual infected plants (55%: Zschau & Janke, 1962; 46%: Beczner & Manninger, 1975), with rates of transmission depending on virus strain and lucerne cultivar or clone (Frosheiser, 1974; Beczner & Manninger, 1975). More seed infection occurs through pollen than through ovules (Frosheiser, 1974; Hemmati & McLean, 1977). Frequency of transmission in lucerne seed is not decreased by storage (Frosheiser, 1974). Seed transmission of 1-5% occurs in chilli pepper (Sûtic, 1959) and of 23% in Nicandra physalodes (Gallo & Ciampor, 1977).
Transmission by Dodder
Occurs in at least five Cuscuta spp. (Schmelzer, 1956).
Serology
Moderately immunogenic, giving antibody titres of 1/1024 (Bancroft et al., 1960). The variously sized intact particles of the virus are serologically indistinguishable. The yellow spot mosaic and 425 strains differ sufficiently from each other for spurs to form in agar gel double-diffusion tests (Van Vloten-Doting, Kruseman & Jaspars, 1968). In the ring interface precipitin test, strains differing widely in pathogenicity and geographical origin behaved as though closely related (Bancroft et al., 1960).
Relationships
No serological relationship to any other virus has been found. The tripartite genome and the presence of the coat protein messenger RNA in the particles (see Particle Composition) suggest a phylogenetic relationship with the bromo- and cucumoviruses, which have isometric particles all of one size, and with ilarviruses such as tobacco streak virus and citrus leaf rugose virus, which have isometric particles of several sizes. The coat proteins of alfalfa mosaic virus and some ilarviruses are needed for infectivity and can reciprocally activate each other’s genomes (Van Vloten-Doting & Jaspars, 1977). Indeed, some workers (Lister & Saksena, 1976; Gonsalves & Fulton, 1977) have suggested that alfalfa mosaic virus should be included in the ilarvirus group. The bacilliform particles of alfalfa mosaic virus have some resemblance to those of a mushroom virus (Hollings, 1962), of a mycoplasma virus (Gourlay, Bruce & Garwes, 1971), of cacao swollen shoot (Brunt, Kenten & Nixon, 1964) and mottle leaf viruses (Kenten & Legg, 1967), and of rubus yellow net virus (Jones & Roberts, 1976). Bacilliform particles have also been observed in preparations of some ilarviruses (Halk & Fulton, 1978).
Stability in Sap
The thermal inactivation point is usually between 60 and 65°C, but may range between 50 and 70°C, the dilution end-point is mostly between 10-3 and 10-4 but is sometimes higher, and the longevity in vitro is usually 1 to 4 days but sometimes considerably longer. Infectivity in sap seems to be best retained at pH 7.0 to 7.5 and phosphate buffers (0.01 to 0.1 M) are used for leaf extraction. Particles are degraded in phosphotungstate stains for electron microscopy except at pH 3 to 4 (Milne & Masenga, 1978).
Purification
Modifications of Steere’s butanol/chloroform method are mostly used (Van Vloten-Doting & Jaspars, 1972) and yield up to 1.5 g virus per kg leaf tissue. Some preparations contain ribosomal material. Alternatively, the virus may be precipitated directly from the sap with polyethylene glycol, M.Wt 20,000, in 0.2 M NaCl (Clark, 1968).
The virus components are separated by differential precipitation in 0.03 M MgSO4, and by centrifugation in sucrose gradients (Van Vloten-Doting & Jaspars, 1972), or by differential solubilization of a polyethylene glycol precipitate (Clark, 1968). Biological activity of purified components often decreases rapidly, but can be stabilized by ethylenediamine-tetraacetate (0.001 M).
Infective preparations of the RNA are best prepared by phenol extraction of the virus in 0.01 M phosphate buffer containing 1% sodium dodecyl sulphate or 2% sodium pyrophosphate. Infectivity of unfractionated RNA, assayed on Phaseolus vulgaris, is about 1% of that of intact virus.
A convenient way to isolate the protein is to dissociate the virus in 0.5 M MgCl2; the RNA precipitates, and the supernatant fluid, after dialysis against 0.05 M acetate buffer, pH 5.5, contains the protein in dimer form (Kruseman et al., 1971).
Properties of Particles
Analytical ultracentrifugation of purified preparations reveals up to six components (Bancroft & Kaesberg, 1960; Hull, 1969), consisting of bacilliform particles of different lengths (Gibbs, Nixon & Woods, 1963). The three larger, obviously bacilliform, components (Fig. 7) are essential for infection and, in order of decreasing size, are named bottom (B), middle (M), and top b (Tb). The two or three spheroidal accessory top components (Ta, To, and Tz) are not infective, singly or together, and cannot replace the function of any of the three larger components of the infective mixture (Van Vloten-Doting, Dingjan-Versteegh & Jaspars, 1970). Infections caused by a mixture of the functional components of two virus strains give pseudo-recombinant strains with properties of both parents. The relative amounts of the components depend on the virus strain and on the growing conditions.
Sedimentation coefficients (svedbergs) at infinite dilution: 94 (B), 82 (M), 73 (Tb), 66 (Ta), c. 60 (To) and c. 53 (Tz).
Molecular weights: 6.9 x 106 (B), 5.2 x 106 (M), 4.3 x 106 (Tb), 3.8 x 106 (Ta).
Electrophoretic mobility: -6.6 x 10-5 cm2 sec-1 volt-1 at pH 7 and 0.1 ionic strength. The virus migrates as a single component, but in sieving media such as 3% polyacrylamide gel it is resolved into at least 17 components. The RNA contributes to the surface charge (Bol & Veldstra, 1969). The virus is precipitated below pH 6. Above pH 4, precipitates retain infectivity for at least a few hours at 4°C.
A260/A280: 1.7-1.8 (B, M, Tb, Ta and probably also the other components).
Absorbance at 260 nm (1 mg/ml, 1 cm light path): 5.1 (B), 4.8 (M and Tb), 4.7 (Ta).
Partial specific volume: 0.703 (B, Ta and probably also the other components).
Buoyant density in Cs2SO4 (fixation with formaldehyde is unnecessary): mean for all components 1.278 g/cm3. Density of the components increases slightly with increasing size (Hull, 1976).
The virus nucleoprotein particles are mainly stabilized by protein-RNA interactions. They are sensitive to low concentrations of sodium dodecyl sulphate (Kaper, 1973), to pancreatic ribonuclease (Pirone, 1962) and trypsin (Bol, Kraal & Brederode, 1974). In the presence of ribonuclease the particles lose RNA fragments and ultimately degrade to smaller structures (Bol & Veldstra, 1969). Particles undergo a reversible unfolding at slightly alkaline pH. Free RNA is capable of removing coat protein subunits from intact nucleoprotein particles (Verhagen et al., 1976). At high ionic strength (e.g. 1.5 M NaCl, pH 5.5) a partially reversible dissociation occurs (Bol & Kruseman, 1969). Nucleoprotein particles have been partially reconstituted from isolated RNA and protein by Lebeurier, Wurtz & Hirth (1969) and Hull (1970a).
Particle Structure
The cylindrical part of the bacilliform particles consists of a hexagonal lattice net (angle of prominent lattice vector with particle axis: 0°; lattice spacing: 4.4 nm). At each end the 6-fold axes of the cylindrical net are probably converted into 5-fold ones giving rise to icosahedral caps. The coat protein subunits are grouped in dimers at the 2-fold positions of the lattice (Mellema, 1975), so that there are 18 subunits per spacing. The components represent a series of particles with 60 + (n x 18) subunits, n being 10 (B), 7 (M), 5 (Tb) and 4 (Ta), respectively (Heijtink, Houwing & Jaspars, 1977). The smaller components To and Tz may have lower n values, but most of these particles have an irregular spheroidal shape. Even in Ta the irregular particles predominate (Heijtink & Jaspars, 1976). Other n values are probably represented by minor components observed upon electrophoresis in polyacrylamide gels (Bol & Lak-Kaashoek, 1974). The lengths (nm) of the purified components (mounted in potassium phosphotungstate after fixation with formaldehyde) are 56 (B), 43 (M), 35 (Tb) and 30 (bacilliform Ta). Particles considerably longer than B are abundant in preparations of strain VRU. In crude preparations of strain 15/64, particles over 1 µm in length have been observed. The diameter of all particles is about 16 nm. With uranyl acetate as negative stain, fixation is unnecessary.
Particle Composition
Nucleic acid: Four species of single-stranded RNA designated RNA 1 to 4, in order of decreasing M. Wt. These are separately packaged in components B (RNA 1), M (RNA 2), Tb (RNA 3) and Ta (2 copies of RNA 4). Minor RNA species of M. Wt intermediate between RNA species 3 and 4 (X-RNA species) or smaller than RNA 4 (Z-RNA species) are found. They are packaged in minor components and in some of the Tb and Ta particles. Particles longer than B do not contain RNA species longer than RNA 1, but have combinations of the above RNA species. M. Wt of RNA-anions: 1.04 x 106, 3250 nucleotides (RNA 1); 0.73 x 106, 2250 nucleotides (RNA 2). 0.62 x 106, 1950 nucleotides (RNA 3); and 283 x 103, 882 nucleotides (RNA 4). The RNA species represent 16.3% (RNA 1), 15.5% (RNA 2 and RNA 3) and 15.2 to 15.6% (RNA 4) of the weight of the corresponding components. Sedimentation coefficients (svedbergs) at infinite dilution (solvent: 0. 01 M sodium phosphate, 0.15 M NaCl, pH 7): 25.8 (RNA 1) and 12.7 (RNA 4). Partial specific volumes (Na salts): 0.459 (RNA 1) and 0.470 (RNA 4). Absorbance at 260 nm (1 mg anion/ml, 1 cm light path, 20°C, 0.01 M sodium phosphate, pH 7): 26.3 (RNA 1) and 25.8 (RNA 4) (Heijtink et al., 1977). Molar percentages of nucleotides: G22.9; A26.8; C20.4; U29.8 for RNA 1 (Rauws, Jaspars & Veldstra, 1964); and G23.9; A24.4; C24.0; U27.7 for RNA 4 (from sequence).
Protein: All components have the same coat protein, which consists of 220 amino acids in strain 425 (M. Wt 24,280). The amino acid sequence is known for strains 425 (Van Beynum et al., 1977), S (Collot et al., 1977) and VRU (Castel et al., 1979). VRU and S proteins have 219 and 217 amino acids, respectively. The N-terminus is acetylated serine. The sequence of the 39 N-terminal amino acids is identical in the three strains. Amino acid compositions of the coat proteins of several strains were compared by Kraal (1975) and protein polymerization behaviour was studied by Driedonks, Krijgsman & Mellema (1977, 1978). Absorbance of protein preparations at 280 nm (1 mg/ml, 1 cm light path, 20°C, 0.05 M sodium acetate, pH 5.5, corrected for light-scattering): 0.70.
Genome Properties
All the RNA species are capped by m7G5' ppp5' Gp (Pinck, 1975) but have no poly(A) tails. No amino acid charging is possible at the 3' ends. RNA species 1, 2 and 3 comprise the complete genome. They share at least 80% sequence homology in the last 150 3'-terminal nucleotides (Pinck & Pinck, 1979; Koper-Zwarthoff et al., 1979; Gunn & Symons, 1980). RNA species 1 and 2 are possibly monocistronic, coding for proteins of M. Wt c. 100,000 and c. 80,000 respectively. RNA 3 is dicistronic, coding for a protein of M. Wt c. 35,000 and for the coat protein (Mohier et al., 1975; Van Vloten-Doting et al., 1977). RNA 4 is homologous with the 3' half of RNA 3 (Gould & Symons, 1978). Little, if any, coat protein is produced by translation of RNA 3 in vitro or in vivo, but RNA 4 is an efficient subgenomic coat protein messenger with a leader sequence of 40 nucleotides (cap and initiation triplet included) (Koper-Zwarthoff et al., 1977). The complete nucleotide sequence is known for RNA 4 of strain 425 (Brederode, Koper-Zwarthoff & Bol, 1980). The leader sequence of RNA 4 has been found to be identical in seven strains (assuming that the cap is the same in all cases), except for position 27 where G, U or A may occur (Swinkels & Bol, 1980).
The ratio RNA 3/RNA 4 in virus preparations is determined by RNA 3. Symptom markers are carried by RNA 2 and RNA 3 (Dingjan-Versteegh, Van Vloten-Doting & Jaspars, 1972; Hartmann et al., 1976). Mutations affecting the relation between growth and temperature were found to be carried by all three genome RNA species (Franck & Hirth, 1976; Van Vloten-Doting et al., 1980). Complementation studies with temperature-sensitive mutants suggest that RNA 1 and RNA 2 each possess more than one gene function. The three genome RNA species and either the coat protein or RNA 4 are needed for infectivity (Bol, Van Vloten-Doting & Jaspars, 1971). Preparations of double-stranded RNA which contain molecules that correspond to each of the genome RNA species have been isolated from infected plants (Mohier, Pinck & Hirth, 1974; Bol et al., 1975).
Relations with Cells and Tissues
Transient, amorphous, granular inclusion bodies have been seen by light microscopy in tobacco leaves (Desjardins, 1966). Electron microscopy has revealed virus particles within the cytoplasm of a number of plants, either non-aggregated or in whorls and rafts containing particles packed side by side (Hull, Hills & Plaskitt, 1969; De Zoeten & Gaard, 1969; Gerola, Bassi & Betto, 1969); similar rafts occur in the vacuoles of tobacco cells (Hull et al., 1969). Particles have also been found in cytoplasmic invaginations in the chloroplasts (Hull, Hills & Plaskitt, 1970; Dingjan-Versteegh, Verkuil & Jaspars, 1974), but not in any organelle, although chloroplasts of basil showed ultrastructural changes (Favali & Conti, 1970). Tobacco and cowpea protoplasts can be infected (Motoyoshi, Hull & Flack, 1975; Alblas & Bol, 1978). Inoculum for cowpea protoplasts must have the same composition as that for intact plants (i.e. complete genome plus either coat protein or RNA 4). Preparations of membrane-bound and solubilized RNA polymerase have been obtained from infected tobacco, but it is uncertain whether virus-coded proteins are involved (Linthorst, Bol & Jaspars, 1980).
Notes
In host range, symptoms, vector relations, nature of the genome and in many other properties alfalfa mosaic virus resembles cucumber mosaic virus. The latter, however, has spherical particles, is independent of coat protein for infection, produces local lesions in Phaseolus vulgaris only in winter, and most strains do not infect Chenopodium amaranticolor or C. quinoa systemically; moreover not all strains of alfalfa mosaic virus infect cucumber.
Figures

Lucerne (alfalfa) with yellow mosaic (above) and vein mosaic (below). (Courtesy L. Beczner, Budapest.)
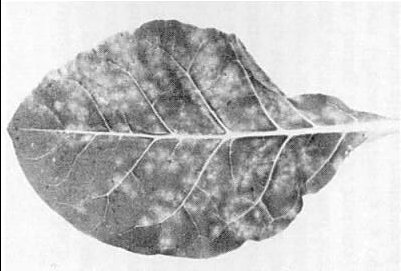
Lesions of strain 425 in inoculated leaf of Nicotiana tabacum cv. White Burley. (Photo IPO, Wageningen.)

Systemic symptoms of strain 425 and recovery in N. tabacum cv. White Burley. (Photo IPO, Wageningen.)

Systemic symptoms of the yellow spot mosaic strain in N. tabacum cv. White Burley. (Photo Dept. Biochem, State Univ., Leiden.)
References list for DPV: Alfalfa mosaic virus (229)
- Alblas & Bol, J. gen. Virol. 14: 653, 1978.
- Bancroft & Kaesberg, Nature, Lond. 181: 720, 1958.
- Bancroft & Kaesberg, Biochim. biophys. Acta 39: 519, 1960.
- Bancroft, Moorhead, Tuite & Liu, Phytopathology 50: 34, 1960.
- Beczner & Manninger, Különlenyomat A Növényvédelmi Kutató Intézet Evkönyve 13: 167, 1975.
- Bol & Kruseman, Virology 37: 485, 1969.
- Bol & Lak-Kaashoek, Virology 60: 476, 1974.
- Bol & Veldstra, Virology 37: 74, 1969.
- Bol, Van Vloten-Doting & Jaspars, Virology 46: 73, 1971.
- Bol, Kraal & Brederode, Virology 58: 101, 1974.
- Bol, Brederode, Janze & Rauh, Virology 65: 1, 1975.
- Brunt, Kenten & Nixon, J. gen. Microbiol. 36: 303, 1964.
- Castel, Kraal, De Graaf & Bosch, Eur. J. Biochem. 102: 125, 1979.
- Clark, J. gen. Virol. 3: 427, 1968.
- Collot, Peter, Das, Wolff & Duranton, Biochim. biophys. Acta 492: 267, 1977.
- Crill, Hagedorn & Hanson, Res. Bull. agric. Exp. Stn Univ. Wis. 280: 40 pp., 1970.
- Desjardins, Phytopathology 56: 875, 1966.
- De Zoeten & Gaard, Virology 39: 768, 1969.
- Dingjan-Versteegh, Van Vloten-Doting & Jaspars, Virology 49: 716, 1972.
- Dingjan-Versteegh, Verkuil & Jaspars, Neth. J. Pl. Path. 80: 72, 1974.
- Driedonks, Krijgsman & Mellema, J. molec. Biol. 113: 123, 1977.
- Driedonks, Krijgsman & Mellema, J. molec. Biol. 124: 713, 1978.
- Favali & Conti, Protoplasma 70: 153, 1970.
- Franck & Hirth, Virology 70: 283, 1976.
- Frosheiser, Pl. Dis. Reptr 54: 591, 1970.
- Frosheiser, Phytopathology 64: 102, 1974.
- Gallo & Ciampor, Acta Virol., Prague 21: 344, 1977.
- Gerola, Bassi & Betto, Protoplasma 67: 307, 1969.
- Gibbs & Tinsley, Pl. Path. 10: 61, 1961.
- Gibbs, Nixon & Woods, Virology 19: 441, 1963.
- Gonsalves & Fulton, Virology 81: 398, 1977.
- Gould & Symons, Eur. J. Biochem. 91: 269, 1978.
- Gourlay, Bruce & Garwes, Nature, New Biol. 229: 118, 1971.
- Gunn & Symons, FEBS Lett. 109: 145, 1980.
- Hagedorn & Hanson, Phytopathology 53: 188, 1963.
- Halk & Fulton, Virology 91: 434, 1978.
- Hartmann, Mohier, Leroy & Hirth, Virology 74: 470, 1976.
- Heijtink & Jaspars, Virology 69: 75, 1976.
- Heijtink, Houwing & Jaspars, Biochemistry 16: 4684, 1977.
- Hemmati & McLean, Phytopathology 67: 576, 1977.
- Hollings, Nature, Lond. 196: 962, 1962.
- Hull, Adv. Virus Res. 15: 365, 1969.
- Hull, Virology 40: 34, 1970a.
- Hull, Virology 42: 283, 1970b.
- Hull, Virology 75: 18, 1976.
- Hull, Hills & Plaskitt, J. Ultrastruct. Res. 26: 465, 1969.
- Hull, Hills & Plaskitt, Virology 42: 753, 1970.
- Jones & Roberts, Ann. appl. Biol. 84: 305, 1976.
- Kaper, Virology 55: 299, 1973.
- Kenten & Legg, J. gen. Virol. 1: 465, 1967.
- Koper-Zwarthoff, Lockard, Alzner-deWeerd, RajBhandary & Bol, Proc. natn. Acad. Sci. U.S.A. 74: 5504, 1977.
- Koper-Zwarthoff, Brederode, Walstra & Bol, Nucleic Acids Res. 7: 1887, 1979.
- Kraal, Virology 66: 336, 1975.
- Kruseman, Jaspars, Bol, Brederode & Veldstra, Biochemistry 10: 447, 1971.
- Lebeurier, Wurtz & Hirth, C.r.hebd. Séanc. Acad. Sci. Paris 268D: 2002, 1969.
- Linthorst, Bol & Jaspars, J. gen. Virol. 46: 511, 1980.
- Lister & Saksena, Virology 70: 440, 1976.
- Mellema, J. molec. Biol. 94: 643, 1975.
- Milne & Masenga, Abstr. 32nd int. Congr. Pl. Path. München p. 18, 1978.
- Mohier, Pinck & Hirth, Virology 58: 9, 1974.
- Mohier, Hirth, Le Meur & Gerlinger, Virology 68: 349, 1975.
- Motoyoshi, Hull & Flack, J. gen. Virol. 27: 263, 1975.
- Pinck, FEBS Lett. 59: 24, 1975.
- Pinck & Pinck, FEBS Lett. 107: 61, 1979.
- Pirone, Phytopathology 52: 747, 1962.
- Pirone, Virology 23: 107, 1964.
- Rauws, Jaspars & Veldstra, Virology 23: 283, 1964.
- Ross, Phytopathology 31: 410, 1941.
- Schmelzer, Phytopath. Z. 28: 1, 1956.
- Schmelzer, Schmidt & Beczner, Biol. Zbl. 92: 211, 1973.
- Sûtic, Phytopath. Z. 36: 84, 1959.
- Swenson, Phytopathology 42: 261, 1952.
- Tremaine & Stace-Smith, Phytopathology 59: 521, 1969.
- Van Beynum, De Graaf, Castel, Kraal & Bosch, Eur. J. Biochem. 72: 63, 1977.
- Van Vloten-Doting & Jaspars, Virology 48: 699, 1972.
- Van Vloten-Doting & Jaspars, in Comprehensive Virology, Vol. 11, p. 1, ed. H. Fraenkel-Conrat & R. R. Wagner, New York: Plenum, 1977.
- Van Vloten-Doting, Kruseman & Jaspars, Virology 34: 728, 1968.
- Van Vloten-Doting, Dingjan-Versteegh & Jaspars, Virology 40: 419, 1970.
- Van Vloten-Doting, Bol, Neeleman, Rutgers, Van Dalen, Castel, Bosch, Marbaix, Huez, Hubert & Cleuter, in Nucleic Acids and Protein Synthesis in Plants, p. 387, ed. L. Bogorad & J. H. Weil, New York: Plenum, 1977.
- Van Vloten-Doting, Hasrat, Oosterwijk, Van't Sant, Schoen & Roosien, J. gen. Virol. 46: 415, 1980.
- Verhagen, Van Boxsel, Bol, Van Vloten-Doting & Jaspars, Annls Microbiol. (Paris) 127A: 165, 1976.
- Verhoyen, in Viruses of Plants, p. 22, ed. A. B. R. Beemster & J. Dijkstra, Amsterdam: North-Holland, 1966.
- Weimer, Phytopathology 21: 122, 1931.
- Weimer, Phytopathology 24: 239, 1934.
- Zaumeyer, Phytopathology 53: 444, 1963.
- Zschau & Janke, NachrBl. dt. PflSchutzdienst, Berl. 16: 94, 1962.
- Brederode, Koper-Zwarthoff & Bol, Nucleic Acids Res. 8: 2213, 1980.
- Swinkels & Bol, Virology, 106: 145, 1980.



