Details of DPV and References
DPV NO: 232 July 1981
Family: Geminiviridae
Genus: Begomovirus
Species: Tobacco leaf curl virus | Acronym:
Tobacco leaf curl virus
T. Osaki College of Agriculture, University of Osaka Prefecture, Sakai, Osaka 591, Japan
T. Inouye College of Agriculture, University of Osaka Prefecture, Sakai, Osaka 591, Japan
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Disease described by Peters & Schwartz (1912), transmission described by
Storey (1931) and Thung (1932); virus characterized by Osaki & Inouye
(1978a).
- Selected synonyms
- Tobacco cabbaging, tobacco curly leaf, and tobacco frenching viruses (Rev.
appl. Mycol. 11: 676)
- Tobacco leaf curl virus 1 (Rev. appl. Mycol. 17: 74)
- Tobacco leaf curl virus 1 (Rev. appl. Mycol. 17: 74)
- A virus with ‘geminate’ particles measuring c. 15-20 x 25-30 nm. It has a fairly wide host range and is transmitted by the whitefly, Bemisia tabaci, in the persistent (circulative) manner, but not by inoculation with sap. Occurs widely in tropical and sub-tropical countries.
Main Diseases
In tobacco, the virus causes stunting; the stems are twisted and the leaves are small, curled, twisted and puckered, often with green thickenings or enations along the veins (Fig. 2, Fig. 3). In tomato, the virus causes curling, yellowing and puckering of the leaves (Fig. 4). In pepper, it causes curling, rolling, puckering, blistering of the leaves and swelling of the veins. Many other types of symptom in tobacco (Thung, 1932; Pal & Tandon, 1937; Wolf, Whitcomb & Moony, 1949; McClean, 1940), tomato (Vasudeva & Sam Raj, 1948; Yassin & Nour, 1965; Osaki, Kobatake & Inouye, 1976) and pepper (Mishra, Raychaudhuri & Jha, 1963; Seth & Dhanraj, 1972; Sugiura, Bandaranayake & Hemachandra, 1975) are assumed to be caused by strains of the virus, although there is little serological evidence that the causal viruses are related. Severity of symptom expression may depend on the strain or mixture of strains present. In Japan, honeysuckle (Lonicera japonica) shows vein yellowing symptoms (Fig. 1) and/or enations, and is the possible perennial reservoir host of the virus (Osaki, Kobatake & Inouye, 1979).
Geographical Distribution
Occurs mainly in tropical and sub-tropical regions, but is also reported in temperate regions e.g. Japan and parts of Europe and USA.
Host Range and Symptomatology
The virus has not been transmitted by inoculation with sap. In transmissions using the vector, the experimental host range is rather narrow, comprising species from Solanaceae, Compositae and Caprifoliaceae (Sugiura et al., 1975; T. Osaki, unpublished data), but plants in five other families have been reported naturally infected. Natural hosts include Ageratum conyzoides (Pruthi & Samuel, 1942), Carica papaya (Nariani, 1956), Eupatorium odoratum, Euphorbia hirta (Pruthi & Samuel, 1942), Lonicera japonica (Osaki et al., 1979), Sida rhombifolia, Solanum nigrum, Vernonia cinerea (Pruthi & Samuel, 1942) and Withania somnifera (Phatak & Raychaudhuri, 1967).
- Diagnostic species
- Datura stramonium.
Clearing of veinlets in young leaves followed by interveinal chlorosis of the lamina in the area between the secondary veins. Stunting of plants accompanied by dwarfing, curling and spiral twisting of the leaves (Fig. 5). - Lycopersicon esculentum (tomato). Yellowing and curling of the young
leaves is usually the first sign of infection.
Leaflets become yellow but remain green along the veins; they are curled and
puckered, and remain small
(Fig. 4). The plant becomes markedly stunted. Some isolates cause vein thickenings
or enations on the veins
(Vasudeva & Sam Raj, 1948; Yassin & Nour, 1965).
- Nicotiana glutinosa. Curling, crinkling and dwarfing of leaves. Sometimes small enations or green thickenings may develop on the larger veins. Some isolates are not transmissible to N. glutinosa (Sugiura et al., 1975).
- Nicotiana glutinosa. Curling, crinkling and dwarfing of leaves. Sometimes small enations or green thickenings may develop on the larger veins. Some isolates are not transmissible to N. glutinosa (Sugiura et al., 1975).
- Propagation and assay species
- Datura stramonium, Lycopersicon esculentum, Nicotiana glutinosa
or other susceptible solanaceous plants are used for propagation and assay of the virus. No local lesion host is known.
Strains
Various isolates of the virus differing in host range or severity of symptoms produced in tobacco have been reported. Most isolates consist of a mixture of strains or variants (Pal & Tandon, 1937; McClean, 1940; Vasudeva & Sam Raj, 1948; Yassin & Nour, 1965; Sugiura et al., 1975; T. Osaki, unpublished data), and some isolates lost ability to cause severe leaf curl symptoms on tobacco after passage through tomato or tobacco (McClean, 1940; Vasudeva & Sam Raj, 1948; T. Osaki, unpublished data). An isolate from tomato in Japan (T. Osaki, unpublished data) causes milder symptoms in tomato than other isolates, and produces no symptoms in tobacco; it did not change in virulence after serial passage in tobacco.
Transmission by Vectors
The whitefly Bemisia tabaci is the most important vector (Pruthi & Samuel, 1937; Bird & Maramorosch, 1978). Vector whiteflies can transmit the virus after a 30 min acquisition access period and a 60 min inoculation access period (Sugiura et al., 1975; Osaki, Kobatake & Inouye, 1977).
The minimum latent period in the vector is between 4 and 9 h, depending on the
length of the acquisition access period (Sugiura et al., 1975; Osaki
et al., 1977). The virus is retained by the vector for more than 12 days
(Sugiura et al., 1975), 16 days (Osaki et al., 1977) or life
(Varma, 1963). There is no evidence that the virus multiplies in the vector.
Transmission through Seed
Not found.
Transmission by Dodder
Not transmitted by Cuscuta campestris (T. Osaki, unpublished data).
Serology
Moderately immunogenic. An antiserum prepared by intramuscular injection using purified virus had titres of 1/256 and 1/32 in microprecipitin and immunodiffusion tests respectively (Osaki & Inouye, 1978b). No serological differences were detected between Japanese isolates of the virus from tomato and tobacco propagated in the same host species. However, spurs formed in immunodiffusion tests between preparations of the same virus isolate propagated in different hosts (Osaki & Inouye, 1980).
Relationships
Tomato isolates of the virus collected from Japan and Taiwan are serologically related (S. K. Green, personal communication). No serological relationship was found among four viruses that belong to the geminivirus group: tobacco leaf curl, maize streak, cassava latent and bean golden mosaic (Osaki & Inouye, 1978 b; K. R. Bock and G. E. Galvez, personal communications).
Stability in Sap
No information.
Purification
Homogenize infected tissues in 0.2 M borate buffer (pH 8.5) containing 0.1% 2-mercaptoethanol and 1% Antifoam A emulsion (2 ml buffer/g tissue). Squeeze the sap through cheesecloth and clarify by adding n-butanol to 10% (v/v) and centrifuging at low speed, retaining the supernatant fluid. Precipitate the virus by adding polyethylene glycol (PEG), M. Wt 6000, to 4% and centrifuging at 10,000 g for 10 min. Resuspend the sediment in 0.01 M potassium phosphate buffer (pH 7.4) and clarify by centrifugation at 10,000 g for 15 min. Layer the supernatant fluid over 10% sucrose and centrifuge at 100,000 g for 2 h. Resuspend the sediment. Further purification can be achieved by centrifugation in reverse concentration PEG solubility gradients, followed by sucrose density gradient centrifugation (Osaki & Inouye, 1978a).
Properties of Particles
Purified preparations produce a single light-scattering band in sucrose density gradient centrifugation (Osaki & Inouye, 1978a).
A260/A280: c. 1.4 (not corrected for
light-scattering) (Osaki & Inouye, 1978a).
Particle Structure
Particles are geminate structures measuring 15-20 x 25-30 nm (Fig. 6, Fig. 7); they consist of two incomplete T = 1 icosahedral structures, with one shared subunit (Osaki & Inouye, 1979). The particles disintegrate in negative stains unless previously fixed with 2% glutaraldehyde (Osaki & Inouye, 1978a).
Particle Composition
Nucleic acid: Electron microscopy of the virus nucleic acid revealed small circular molecules; this suggests that the virus particles, like those of some other geminiviruses, contain circular DNA molecules (T. Osaki, unpublished data).
Protein: No information.
Relations with Cells and Tissues
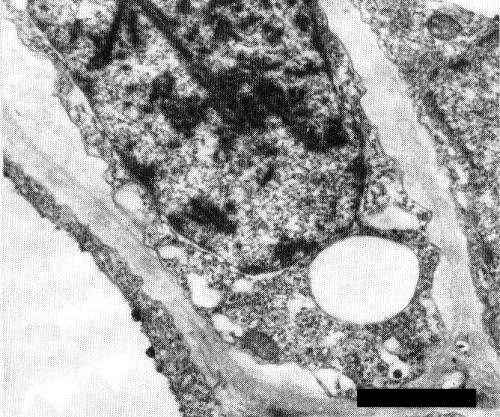
Phloem proliferation occurs in stem apices, petioles, and leaflet veins and veinlets (Kerling, 1933; Yassin & Nour, 1965). In ultrathin sections of infected tissues of tomato, Datura stramonium, Nicotiana glutinosa and Lonicera japonica, virus-like particles occur in close-packed arrays forming rigid rod structures in some of the nuclei in phloem cells (Fig. 9). The rods, each of which is c. 25 nm wide, consist of a row of geminate particles (Fig. 8) (Osaki & Inouye, 1978a). A distinctive abnormality of the nuclei is the splitting of the nucleoli into granular and fibrillar regions (Fig. 10) (Osaki et al., 1979).
Notes
The virus may be distinguished from two other whitefly-borne viruses with geminate particles, tomato yellow leaf curl (Cohen & Nitzany, 1966; Russo, Cohen & Martelli, 1980) and tomato golden mosaic (Matyis et al., 1975) because tomato yellow leaf curl virus has a much longer period of latency (21 h at least) and progressively loses infectivity in the vector whitefly (Cohen & Nitzany, 1966), and tomato golden mosaic virus is transmissible by inoculation with sap (Costa, 1976). The virus may also be distinguished from other Bemisia tabaci-transmitted viruses that infect solanaceous plants, such as tomato yellow mosaic virus in Venezuela or India, because they cause mosaic in tomato and tobacco (De Uzcategui & Lastra, 1978; Verma, Srivastava & Mathur, 1975). However, information on the interrelationships of these viruses is still fragmentary and further work to compare them serologically and in other ways is desirable.
Figures
References list for DPV: Tobacco leaf curl virus (232)
- Bird & Maramorosch, Adv. Virus Res. 22: 55, 1978.
- Cohen & Nitzany, Phytopathology 56: 1127, 1966.
- Costa, A. Rev. Phytopath. 14: 429, 1976.
- De Uzcategui & Lastra, Phytopathology 68: 985, 1978.
- Kerling, Phytopathology 23: 175, 1933.
- McClean, Sci. Bull. Dept. Agric. For. Un. S. Afr. 225: 1, 1940.
- Matyis, Silva, Oliveira & Costa, Summa Phytopath. 1: 267, 1975.
- Mishra, Raychaudhuri & Jha, Indian J. Microbiol. 3: 73, 1963.
- Nariani, Indian Phytopath. 9: 151, 1956.
- Osaki & Inouye, Ann. Phytopath. Soc. Japan 44: 167, 1978a.
- Osaki & Inouye, Ann. Phytopath. Soc. Japan 44: 385, 1978b.
- Osaki & Inouye, Ann. Phytopath. Soc. Japan 45: 573, 1979.
- Osaki & Inouye, Ann. Phytopath. Soc. Japan 46: 92, 1980.
- Osaki, Kobatake & Inouye, Shokubutsu Boeki 30: 458, 1976.
- Osaki, Kobatake & Inouye, Ann. Phytopath. Soc. Japan 43: 372, 1977.
- Osaki, Kobatake & Inouye, Ann. Phytopath. Soc. Japan 45: 62, 1979.
- Pal & Tandon, Indian J. agric. Sci. 7: 363, 1937.
- Peters & Schwartz, Mitt. K. biol. Anst. Ld-u. Fortw. 7, 198 pp., 1912.
- Phatak & Raychaudhuri, Sci. Cult. 33: 223, 1967.
- Pruthi & Samuel, Indian J. agric. Sci. 7: 659, 1937.
- Pruthi & Samuel, Indian J. agric. Sci. 12: 37, 1942.
- Russo, Cohen & Martelli, J. gen. Virol. 49: 209, 1980.
- Seth & Dhanraj, Phytopath. Z. 73: 365, 1972.
- Storey, Nature, Lond. 128: 187, 1931.
- Sugiura, Bandaranayake & Hemachandra, Tech. Bull. trop. Agric. Res. Cent. 8: 1, 1975.
- Thung, Meded. Proefstn vorstenl. Tabak Klaten 74: 1, 1932.
- Varma, Bull. natn. Inst. Sci. India 24: 11, 1963.
- Vasudeva & Sam Raj, Phytopathology 38: 364, 1948.
- Verma, Srivastava & Mathur, Pl. Dis. Reptr 59: 494, 1975.
- Wolf, Whitcomb & Moony, J. Elisha Mitchell scient. Soc. 65: 38, 1949.
- Yassin & Nour, Ann. appl. Biol. 56: 207, 1965.