Details of DPV and References
DPV NO: 239 July 1981
Family: Potyviridae
Genus: Macluravirus
Species: Maclura mosaic virus | Acronym: MacMV
Maclura mosaic virus
Renate Koenig Institut für Viruskrankheiten der Pflanzen, Biologische Bundesanstalt für Land- und Forstwirtschaft, D 3300 Braunschweig, Germany
D.-E. Lesemann Institut für Viruskrankheiten der Pflanzen, Biologische Bundesanstalt für Land- und Forstwirtschaft, D 3300 Braunschweig, Germany
Nada Plese Department of Botany, Faculty of Science, University of Zagreb, Yu 41000 Zagreb Yugoslavia
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by Plese & Milicic (1973).
A virus with filamentous particles 672 x 13-16 nm causing mosaic in Maclura pomifera and infecting a few species from other families. It induces pinwheel inclusions in infected cells, is transmitted by aphids in a non-persistent manner and is sap-transmissible. Found in Yugoslavia.
Main Diseases
Causes interveinal mosaic and sometimes vein-banding and leaf distortion in Maclura pomifera (osage orange), especially on leaves developing in spring (Fig. 1) (Plese & Milicic, 1973; Plese & Stefanac, 1976).
Geographical Distribution
Recorded only from Yugoslavia.
Host Range and Symptomatology
Transmitted experimentally by inoculation of sap to young seedlings of Maclura pomifera (Moraceae) and to a rather narrow range of herbaceous hosts comprising 17 species in 5 dicotyledonous families (Plese & Stefanac, 1976; Plese et al., 1979). Most of the susceptible species are in the Chenopodiaceae and Solanaceae. Many hosts are infected only locally and develop mild symptoms or remain symptomless.
-
Diagnostic species
- Maclura pomifera
(osage orange). Young seedlings develop systemic mosaic similar to that in naturally infected plants (Fig. 1) c. 3 weeks after mechanical inoculation. - Chenopodium amaranticolor. Distinct circular necrotic local lesions about 1-2
mm in diameter after 1 week
(Fig. 2); no systemic infection.
- Tetragonia expansa. Diffuse chlorotic local lesions after 1 week, slowly enlarging and coalescing (Fig. 3); no systemic infection.
- Nicotiana clevelandii. Inoculated leaves are usually symptomless. Systemically infected leaves develop mild mosaic in 10-14 days.
- Tetragonia expansa. Diffuse chlorotic local lesions after 1 week, slowly enlarging and coalescing (Fig. 3); no systemic infection.
-
Propagation species
- Tetragonia expansa
is suitable as a source of inoculum. Nicotiana clevelandii is a good source of virus for purification.Assay species
- Chenopodium amaranticolor
is a useful test plant for local lesion assay and for aphid transmission tests.
Strains
None recorded.
Transmission by Vectors
Transmitted in a non-persistent manner by the aphid Myzus persicae (Plese & Stefanac, 1976). Aphids acquire and inoculate virus in 5-10 min feeding periods.
Transmission through Seed
Not studied.
Serology
Moderately immunogenic, antisera with precipitin titres of 1/512 were obtained (Plese et al., 1979).
Precipitates in liquid media are floccular.
Relationships
No unequivocal serological relationship was found with any other elongated virus, including members of the potyvirus, carlavirus and closterovirus groups (Plese et al., 1979). Although maclura mosaic virus induces ‘pinwheel’ inclusions in infected cells, it differs from typical potyviruses in its high protein M. Wt, short particle length and some other particle properties (Plese et al., 1979).
Stability in Sap
In Tetragonia expansa sap, the virus has a thermal inactivation point (10 min) of 65°-67°C, dilution end-point of 10-3-10-4, and retains infectivity for at least 3 days at 20°C.
Purification
Homogenize 100 g leaves from systemically infected Nicotiana clevelandii in 100 ml 0.5 M borate buffer, pH 7.8, containing 0.2% ascorbic acid and 0.2% sodium sulphite. Stir the supernatant fluid obtained after low speed centrifugation with 0.4 vol. chloroform. Break the emulsion by low speed centrifugation. Sediment and concentrate the virus from the aqueous phase by two cycles of high and low speed centrifugation. Further purify by sucrose density gradient centriflugation (Plese et al., 1979).
Properties of Particles
Purified preparations contain a single sedimenting component. Sedimentation
velocity (relative to marker viruses): 155 S
(Plese et al., 1979).
A260/A280: 1.04 (corrected for light-scattering)
(Plese et al., 1979).
Buoyant density: 1.307 g/cm3 in CsCl (Plese et al., 1979).
Particle Structure
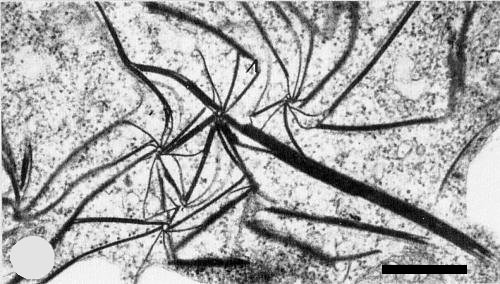
Particles are elongated and flexuous (Fig. 4) with a modal length of 672 nm (Plese et al., 1979). The width of the particles is 13-16 nm (D.-E. Lesemann, unpublished data). Thus the particles are shorter and thicker than those of definitive potyviruses. Particles negatively stained in uranyl salts show an indistinct cross-banding pattern (Fig. 5). In some samples of crude sap negatively stained with sodium phosphotungstate the virus particles are surrounded by a regular pattern of granules of unknown material (Fig. 6).
Particle Composition
Nucleic acid: 3-4% of the particle weight (estimated from the A260/A280 ratio) (Plese et al., 1979).
Protein: Single species with M. Wt c. 4.5 x 104 (Plese et al., 1979).
Relations with Cells and Tissues
Amorphous granular cytoplasmic inclusions were detected in infected Tetragonia expansa cells (Fig. 7) by light microscopy (Plese & Wrischer, 1978). All host plants studied by electron microscopy of ultrathin sections contained cylindrical cytoplasmic inclusions (Plese & Stefanac, 1976; Plese et al., 1979). These are visible as pinwheels and laminated aggregates (Fig. 8), typical of viruses in subdivision II of the potyviruses as defined by Edwardson (1974). No tubes or scrolls were seen. Filaments about 20 nm thick (possibly virus particles) were found to be closely associated with the pinwheel plates and laminated aggregates.
Notes
Maclura mosaic disease is readily distinguished from maclura ringspot which also occurs in Yugoslavia and is apparently caused by cucumber mosaic virus (Plese & Milicic, 1973).
Figures

Particles of maclura mosaic virus from a purified preparation negatively stained with 2% uranyl acetate. Bar represents 200 nm.
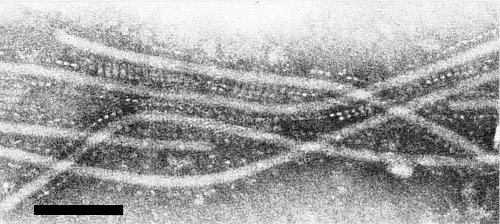
Particles with associated granular structures from crude sap of Nicotiana clevelandii negatively stained with sodium phosphotungstate, pH 7.0. Bar represents 100 nm.
References list for DPV: Maclura mosaic virus (239)
- Edwardson, Monograph. Ser. Fla agric. Exp. Stn 4, 398 pp., 1974.
- Plese & Milicic, Phytopath. Z. 77: 178, 1973.
- Plese & Stefanac, Mitt. biol. BundAnst. Ld- u. Forstw. 170: 47, 1976.
- Plese & Wrischer, Acta bot. croat. 37: 47, 1978.
- Plese, Koenig, Lesemann & Bozarth, Phytopathology 69: 471, 1979.