Details of DPV and References
DPV NO: 242 July 1981
Family: Potyviridae
Genus: Potyvirus
Species: Potato virus Y | Acronym: PVY
This is a revised version of DPV 37
Potato virus Y
J. A. de Bokx Research Institute for Plant Protection, Wageningen, The Netherlands
H. Huttinga Research Institute for Plant Protection, Wageningen, The Netherlands
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by Smith (1931).
- Selected synonyms
- Potato acropetal necrosis virus (Rev. appl. Mycol. 10: 745)
- Potato severe mosaic virus (Rev. appl. Mycol. 22: 367)
- Marmor upsilon (Rev. appl. Mycol. 28: 514)
- Solanum virus 2 (Rev. appl. Mycol. 17: 52)
- Tobacco vein-banding virus (Rev. appl. Mycol. 10: 60)
- Potato severe mosaic virus (Rev. appl. Mycol. 22: 367)
- The following are names of strains:
- Potato virus C (Rev. appl. Mycol. 16: 53)
- Tobacco veinal necrosis virus (Rev. appl. Mycol. 31: 201)
- Tobacco veinal necrosis virus (Rev. appl. Mycol. 31: 201)
- A virus with long flexuous particles 730 x 11 nm, helically constructed, with a pitch of 3.3 nm. Particles contain about 6% single-stranded RNA and a protein of M. Wt 34,000. The virus is easily transmitted mechanically to a narrow range of hosts and is transmitted by many aphid species in the non-persistent manner. Occurs world-wide and causes economically important diseases in several solanaceous crops.
Main Diseases
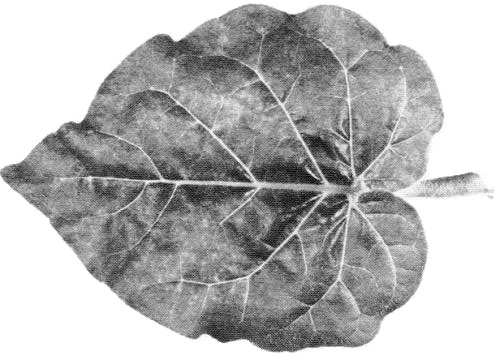
In potato the virus may cause (a) mild to severe mottle, (b) streak or ‘leaf-drop streak’ (Fig. 1) with necrosis along the veins of the underside of the leaflets, (c) ‘stipple-streak’ (Beemster & Rozendaal, 1972).
YN strains may cause necrotic rings or spots on the first infected leaf of some potato cultivars but symptoms of primary (first year) infections are usually very slight; mild to very mild mottle may appear late in the growing season. Secondary symptoms (those that appear in the second and subsequent years) are usually more obvious, taking the form of mild or severe mottle, and may appear early in the growing season depending on climatic conditions. Necrosis is rare.
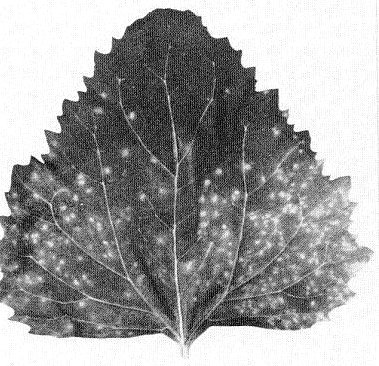
The primary symptoms of YO strains, depending on the potato cultivar, are necrosis, mottling or yellowing of leaflets, leaf-dropping, and sometimes premature death. Necrosis, which starts as spots or rings on the leaflets, may cause leaves to collapse and either drop from the plants or remain hanging from the stem (‘leaf-drop streak’). Secondarily infected plants are dwarfed and brittle, with leaves crinkled and puckered (Fig. 2). Sometimes necrosis occurs in the foliage and stems. Necrosis is usually more severe after primary than after secondary infection. YO strains in mixed infections with potato virus X cause a very destructive disease called ‘rugose mosaic’ in the USA. In Britain, the term ‘rugose mosaic’ is used to refer to disease caused by potato virus Y in the second and subsequent years of infection.
The primary symptoms of YC strains are necrosis, mottling, crinkle or ‘stipple streak’ (necrotic lesions and streaks on leaves and variable streaking on petioles and stems), depending on the potato cultivar. Secondary symptoms are stipple streak, crinkle or mottle. Necrosis may occur in tubers.
The differences between the primary and secondary symptoms induced by potato virus Y are often indistinct because of the diversity of potato cultivars and virus strains. Moreover, symptom expression is affected by climatic conditions (De Bokx & Piron, 1977). Potato virus Y decreases yield of infected potato plants from 10 to 80%, depending on the virus strain, potato cultivar and time of infection: it is transmitted to all tubers of plants with secondary infection.
Most strains of potato virus Y typically cause mild mottling in pepper (Capsicum spp.), tobacco and tomato. Early infection of tobacco with potato virus Y may decrease yield about 30% (Sievert, 1978). However, the tobacco veinal necrosis disease, caused by YN strains, is very destructive and may cause a complete loss of crop. In pepper and tomato heavy losses may occur, especially when potato virus Y is present together with other viruses (Edwardson, 1974a).
Geographical Distribution
YO strains are spread worldwide; YN strains occur in Europe (including USSR), parts of Africa and South America. YC strains (including potato virus C) are probably present in Australia, India, and in some parts of UK and continental Europe. The virus occurs worldwide in potato, and in outdoor crops of pepper, tobacco and tomato in warmer countries.
Host Range and Symptomatology
The host range is mainly limited to the Solanaceae but some members of the Amaranthaceae, Chenopodiaceae, Compositae and Leguminosae are susceptible. The virus has been transmitted by inoculation with sap to about 120 species (Edwardson, 1974b; Horváth, 1979).
- Diagnostic species
- Datura stramonium
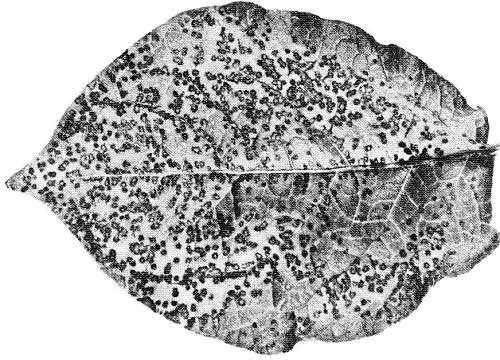
. Immune to infection by all tested strains. - Nicotiana glutinosa. Shows mild to severe mottling, depending on strain, 2 to 3 weeks
after inoculation (Fig. 3).
- N. tabacum (tobacco). All strains except YN strains induce vein-clearing about 2 weeks after inoculation followed by mottling which may later disappear. Cvs Samsun NN and White Burley are useful for differentiating YO and YN strains. YN strains induce vein-clearing and slight epinasty, followed by scattered whitish or brownish necrotic-lesions. The areas of necrosis follow the main veins. Primary veins that are almost fully developed become brown. The leaves collapse prematurely against the stem, only a small bunch of leaves remaining at the top of the plant. Stems may show necrosis near the base (Klinkowski & Schmelzer, 1960).
- Solanum demissum ‘A’. Most strains of potato virus Y give no local lesions. This distinguishes the virus from potato virus A which induces necrotic local lesions 3 to 5 days after inoculation (Cockerham, 1958).
- Solanum tuberosum (potato).YC strains induce local lesions on detached leaves of cv. Duke of York (Eersteling), whereas YN and YO strains do not (J. A. de Bokx & C. Cuperus, unpublished data). Potato cultivars with extreme resistance to potato virus X, e.g. cv. Saco, can be used for separating potato virus Y from potato virus X.
- Tinantia erecta. Displays severe systemic mottling. Immune to potato aucuba mosaic virus and potato virus M and potato virus S (Horváth, 1979).
- N. tabacum (tobacco). All strains except YN strains induce vein-clearing about 2 weeks after inoculation followed by mottling which may later disappear. Cvs Samsun NN and White Burley are useful for differentiating YO and YN strains. YN strains induce vein-clearing and slight epinasty, followed by scattered whitish or brownish necrotic-lesions. The areas of necrosis follow the main veins. Primary veins that are almost fully developed become brown. The leaves collapse prematurely against the stem, only a small bunch of leaves remaining at the top of the plant. Stems may show necrosis near the base (Klinkowski & Schmelzer, 1960).
- Propagation species
- Nicotiana tabacum
cv. Samsun NN is a good source of virus for purification (Huttinga, 1973).- Assay species
- Chenopodium amaranticolor
(Fig. 4), C. quinoa, Physalis floridana, Solanum tuberosum (cvs Duke of York, Saco) and some species of Lycium are suitable local-lesion hosts for some strains. S. chacoense (TE1) (De Bokx, 1974), S. demissum ‘Y’ and S. demissum x S. tuberosum ‘A6’ (Fig. 5) produce necrotic local lesions after inoculation with almost all strains (De Bokx, Kratchanova & Maat, 1975). Local lesions usually appear 5 to 7 days after inoculation.
Strains
Several groups of strains can be distinguished according to the systemic and local symptoms on Nicotiana tabacum (cvs White Burley and Samsun NN), Physalis floridana, Solanum tuberosum cv. Duke of York (De Bokx, 1961; De Bokx & Piron, 1978) and other potato cultivars. The most important groups are:
Potato virus YO group (common strains). Induce mainly severe systemic crinkle symptoms, rugosity or leaf-drop streak in potato, systemic necrosis in Physalis floridana, and systemic mottling in tobacco.
Potato virus YN group (tobacco veinal necrosis strains). Induce severe systemic veinal necrosis in tobacco, systemic mottling in Physalis floridana (Fig. 6) and very mild mottling in almost all potato cultivars.
Potato virus YC group (stipple streak strains, including potato virus C). Potato virus C itself is not transmitted by the aphid Myzus persicae (Watson, 1956), but other strains of this group are (De Bokx & Piron, 1978). Many potato cultivars are hypersensitive to strains in this group (Calvert, Cooper & McClure, 1980). Susceptible cultivars may show systemic mosaic or stipple streak. Symptoms in tobacco and Physalis floridana are similar to those in duced by YOstrains.
Some strains belong to none of these groups (Leiser & Richter, 1979).
Transmission by Vectors
Transmitted by inoculation with sap, by stem and core grafting, and by at least 25 species of aphid in the non-persistent manner. Myzus persicae is the most efficient vector; others are Aphis fabae, Macrosiphum euphorbiae, Myzus certus, Phorodon humuli and Rhopalosiphum insertum (Kennedy, Day & Eastop, 1962; Van Hoof, 1980). Pre-acquisition fasting periods from 15 min up to 1 h, sometimes 3 to 4 h, increase the frequency of transmission. Optimal acquisition feeding time is from 15 to 60 sec; periods lasting more than 2 min are less favourable. Most feeding aphids cease to transmit the virus within 1 h after the end of the acquisition feed, but starved aphids retain it much longer (Watson & Roberts, 1939), some for as long as 24 h. The virus does not pass through the moult. The minimum inoculation feeding time is from 30 to 60 sec (Bradley & Rideout, 1953). The availability of potato virus Y to aphids is correlated with the virus concentration in the plant (Bagnall & Bradley, 1958; De Bokx, Van Hoof & Piron, 1978). Plants infected with potato virus Y contain ‘helper component’, rather labile proteinaceous material of M. Wt 1 to 2 x 105, which mediates transmission of the virus by aphids and also mediates transmission of potato aucuba mosaic virus and potato virus C (Govier & Kassanis, 1974).
Transmission through Seed
No information.
Serology
Strongly immunogenic. Antisera with precipitin titres of 1/512 to 1/4096 have been produced in rabbits injected with purified virus preparations emulsified with Freund’s incomplete adjuvant. The enzyme-linked immunosorbent assay (ELISA) can detect potato virus Y in plants in very low concentrations (Gugerli & Gehringer, 1980). Immunodiffusion tests may be done in agar gels using degraded virus as antigen. Diffusible components of the virus can be obtained by treating it with dissociating agents such as ethanolamine-hydrochloride at pH 10.5 (Purcifull & Gooding, 1970) or sodium dodecyl sulphate (SDS) (Purcifull & Batchelor, 1977). The products of these treatments may react only with antiserum prepared against dissociated virus. The method has proved suitable for diagnosis, for detection of potato virus Y in crude plant extracts, and for determination of serological relationships.
Relationships
Strains of potato virus Y, although closely serologically related, exhibit considerable serological variability (Calvert et al., 1980); however, there are no consistent serological differences between the YO, YN and YC groups. Potato virus Y is serologically distantly related to tobacco etch, henbane mosaic and potato A viruses, although the degree of relationship among these four is difficult to assess (Bartels, 1964). Several other viruses with flexuous particles of modal length 730 to 760 nm, such as pepper veinal mottle (Brunt, Kenten & Phillips, 1978) and bidens mottle viruses (Purcifull, 1976) are distantly serologically related to potato virus Y (Fraenkel-Conrat, 1974).
Bawden & Kassanis (1951) found that a YN strain did not protect tobacco or potato plants from infection by YO or YC strains, and tobacco infected with either virus YO or YC strains was susceptible to a YN strain. Schmelzer, Bartels & Klinkowski (1960) found that a German YN strain (M3) did not protect Samsun tobacco from infection with isolates of strain YO, and in some tests no cross protection was observed even between two YN isolates. However, Todd (1961) reported that YO strains protected against YN strains in tobacco. In further cross-protection tests, several YN strains protected against each other in tobacco (Aubert, 1959) as did some YO strains in potato (De Bokx et al., 1975).
Stability in Sap
In tobacco sap, the thermal inactivation point (10 min) is between 50 and 62°C, the dilution end point between 10-2 and 10-6, and the longevity in vitro (18-22°C) is 7 to 50 days.
Purification
The main difficulty in purifying potato virus Y is aggregation of the particles. Many procedures have been reported to be helpful in avoiding this, but none has been universally applicable. Good results have been obtained with methods reported by Damirdagh & Shepherd (1970), Huttinga (1973), McDonald, Beveridge & Bancroft (1976), Moghal & Francki (1976) and Leiser & Richter (1978). The highest yield reported is 40 mg virus per kg leaves (McDonald et al., 1976; Leiser & Richter, 1978).
McDonald et al. (1976): Homogenize 100 g leaf tissue in 100 ml 50 mM sodium phosphate buffer, pH 7.6, containing 10 mM EDTA and 0.5% (v/v) 2-mercaptoethanol. Squeeze the homogenate through cheesecloth, and centrifuge for 30 min at 12,000 g. Layer the supernatant fluid over a cushion of 6 ml 30% (w/v) sucrose in 50 mM sodium phosphate, pH 7.6, containing 10 mM EDTA (buffer) in a Beckman R30 tube and centrifuge for 3 h at 27,000 rev./min. Resuspend the pellets in 2.5 ml buffer and clarify the suspension by centrifugation at 4000 g for 10 min. Layer 1 ml aliquots over 4 ml CsCl (r = 1.27) in 50 mM potassium phosphate, pH 7.6, centrifuge for 17 h at 36,000 rev./min. in a Beckman SW50.1 rotor and recover the virus zone by puncturing the bottom of the tube.
Leiser & Richter (1978): Homogenize 100 g leaf tissue in 200 ml 0.5 M sodium citrate buffer, pH 7.4, containing 5 mM EDTA and 15 mM sodium DIECA. Squeeze the homogenate through cheesecloth and centrifuge for 15 min at 6000 rev./min. Add Triton X-100 to a final concentration of 3% (v/v) and stir for 30 min at 4°C. Centrifuge for 2 h at 30,000 g. Resuspend the pellets in 10 mM sodium citrate buffer, pH 7.4, containing 1 M urea and 0.1% (v/v) 2-mercaptoethanol. Centrifuge for 15 min at 15,000 rev./min. Layer the supernatant fluid over a cushion of 20% (w/v) sucrose and centrifuge for 2 h at 50,000 g. Resuspend the pellets in 5 mM borate buffer, pH 8, in 30 mM NaCl, 3 mM sodium citrate. To improve yield the sediments of the centrifugations for 15 min at 15,000 rev./min are extracted once more before being discarded.
Properties of Particles
Purified preparations contain a single infective sedimenting component.
Sedimentation coefficient at infinite dilution in 0.1 M Tris-HCl, pH 9, at 20°C: 145 S (Huttinga, 1975).
Buoyant density in CsCl: 1.323 g/cm3 for a YO strain and 1.326 g/cm3 for a YN strain (Huttinga, 1975).
A260/A280 (corrected for light-scattering): 1.21-1.22 (Leiser & Richter, 1978).
Absorbance at 260 nm (1 mg/ml, 1 cm light path): 2.3, corrected for light-scattering (Leiser & Richter, 1978); 2.9, not corrected for light-scattering (Stace-Smith & Tremaine, 1970; Leiser & Richter, 1978).
Particle Structure
The virus particles are flexuous filaments (Fig. 7) about 11 nm wide and helically constructed, with a pitch of about 3.3 nm (Varma et al., 1968). Delgado-Sanchez & Grogan (1966) reported that the modal length of the particles in purified preparations is about 684 nm, compared with about 730 nm for particles in dip preparations. De Bokx, Kratchanova & Maat (1975) reported a modal length of about 740 nm for particles in clarified sap of tobacco cv. White Burley. The length and flexuousness of the particles of potato virus Y are unaffected by Mg++ or EDTA (Govier & Woods, 1971), which contrasts with the behaviour of some other potyviruses (Huttinga, 1975).
Particle Composition
Nucleic acid: RNA, single-stranded, M. Wt about 3.1 x 106 as calculated from the sedimentation coefficient (25 S) of formaldehyde-treated RNA (Makkouk & Gumpf, 1974). Hinostroza-Orihuela (1975) determined a M. Wt of 3.2 x 106 by polyacrylamide gel electrophoresis. The RNA comprises 5.4-6.4% of the particle mass (Stace-Smith & Tremaine, 1970; Leiser & Richter, 1978). The RNA in the particles is a positive strand because it is infective by itself (Makkouk & Gumpf, 1974; Hinostroza-Orihuela, 1975).
Protein: Virus coat protein migrates as two bands in SDS-polyacrylamide gels. The M. Wt of the slower migrating polypeptide is 33,000-34,000 (Hiebert & McDonald, 1973; Huttinga & Mosch, 1974; Moghal & Francki, 1976). The faster migrating component has a M. Wt of 28,000 and is probably a breakdown product (Huttinga & Mosch, 1974; Moghal & Francki, 1976). However, Hiebert & McDonald (1973) suggest that the 34,000 M. Wt component is a charge isomer of the 28,000 M. Wt component. Amino-acid compositions have been determined by Stace-Smith & Tremaine (1970), Miki & Oshima (1972), Makkouk & Gumpf (1975) and Moghal & Francki (1976).
Relations with Cells and Tissues
Cylindrical inclusions occur mainly in epidermal tissues. After staining with suitable dyes (Rubio-Huertos, 1972), inclusions can be observed with the light microscope as early as 48 h after infection in such hosts as tobacco, pepper and tomato; data on potato are lacking. The cylindrical cytoplasmic inclusions, depending on the plane of ultrathin sectioning, appear as scrolls or laminated aggregates (Fig. 8) (Christie & Edwardson, 1977). They contain a protein of 67,000 M. Wt, estimated by electrophoresis in SDS-polyacrylamide gels (Hiebert & McDonald, 1973), which is coded for by the viral genome but is serologically distinct from the coat protein (Purcifull, Hiebert & McDonald, 1973). In cytoplasm of Datura metel infected with a YN strain, mitochondria were frequently surrounded by filaments with a diameter of 9 to 10 nm but of indeterminate length (Borges & David-Ferreira, 1968). Crystalline intranuclear inclusions have been reported in tissues infected with some strains of potato virus Y (Kitajima, Camargo & Costa, 1968).
Notes
In some Solanum spp., symptoms of potato virus Y may be confused with those of tobacco etch and henbane mosaic viruses. These viruses may be distinguished from potato virus Y by their ability to infect Datura stramonium, and by their effects on tobacco and ‘Tabasco’ pepper (Delgado-Sanchez & Grogan, 1970). Potato virus A can be distinguished from most strains of potato virus Y (Cockerham, 1958) by the reactions of the local lesion host Solanum demissum ‘A’ (Butzonitch & De Bokx, 1978), and by its failure to infect the potato cultivars Bintje, Duke of York and Red Craigs Royal after mechanical inoculation. These cultivars are field-immune to potato virus A but susceptible to potato virus Y (except that Red Craigs Royal is field-immune to YC strains (Calvert et al, 1980)). They display crinkle or slight mottle after mechanical inoculation with YO or YN strains respectively. Cvs Bintje and Duke of York show stipple streak after inoculation with YC strains.
In certification schemes, detached leaves of S. demissum x S. tuberosum ‘A6’ are commonly used to detect potato virus Y. However, this host is not suitable for identifying which virus is present, because several other viruses induce local lesions in ‘A6’ (Bartels, 1970). In temperate zones, perennials seldom act as virus reservoirs in nature. The potato itself, as ‘ground-keepers’, may be considered a reservoir host (Thresh, 1980). In tropical and subtropical areas, weeds such as Solanum atropurpureum (Chagas et al., 1977) and other Solanum spp. (Edwardson, 1974a) may act as important virus sources.
Attempts to reduce virus spread through vector control by insecticides have been ineffective. The main control methods are: (i) avoidance of infection, i.e. growing crops in periods in which vectors are absent or population density is low; (ii) not growing crops near established crops of the same species; (iii) destroying haulms of seed-potato crops before maturity so as to restrict virus spread at the end of the growing season; (iv) spraying with mineral oils to reduce the frequency of transmission (Bradley, Moore & Pond, 1966; Vanderveken, 1977); (v) breeding for resistance: this is an efficient way to control potato virus Y, if sources of durable resistance can be obtained; (vi) use of reflective surfaces and sticky yellow sheets which can diminish virus spread (Loebenstein & Raccah, 1980).
Figures
References list for DPV: Potato virus Y (242)
- Aubert, Mém. Soc. vaud. Sci. nat. 12: 153, 1959.
- Bagnall & Bradley, Phytopathology 48: 121, 1958.
- Bartels, Phytopath. Z. 49: 257, 1964.
- Bartels, Potato Res. 13: 119, 1970.
- Bawden & Kassanis, Ann. appl. Biol. 38: 402, 1951.
- Beemster & Rozendaal, in Viruses of Potatoes and Seed-potato Production, p. 115, ed. J. A. de Bokx, 233 pp., Wageningen: PUDOC, 1972.
- Borges & David-Ferreira, Revta Biol., Lisb. 6: 421, 1968.
- Bradley & Rideout, Can. J. Zool. 31: 333, 1953.
- Bradley, Moore & Pond, Nature, Lond. 209: 1370, 1966.
- Brunt, Kenten & Phillips, Ann. appl. Biol. 88: 115, 1978.
- Butzonitch & De Bokx, Fitopatologia 13: 77, 1978.
- Calvert, Cooper & McClure, Rec. agr. Res. N. Ireland 28: 63, 1980.
- Chagas, Vicente, Alba & July, Phytopath. Z. 90: 147, 1977.
- Cockerham, Proc. 3rd Conf. Potato Virus Diseases, Lisse-Wageningen: 199, 1958.
- Christie & Edwardson, Monograph Ser. Fla agric. Exp. Stn 9, 150 pp., 1977.
- Damirdagh & Shepherd, Phytopathology 60: 132, 1970.
- De Bokx, Tijdschr. PlZiekt. 67: 273, 1961.
- De Bokx, Potato Res. 17: 323, 1974.
- De Bokx, Kratchanova & Maat, Potato Res. 18: 38, 1975.
- De Bokx & Piron, Potato Res. 20: 207, 1977.
- De Bokx & Piron, Abstr. 7th Trienn. Conf. Eur. Ass. Potato Res., p. 244, 1978.
- De Bokx, Van Hoof & Piron, Neth. J. Pl. Path. 84: 95, 1978.
- Delgado-Sanchez & Grogan, Phytopathology 56: 1397, 1966.
- Delgado-Sanchez & Grogan, CMI/AAB Descriptions of Plant Viruses 37, 4 pp., 1970.
- Edwardson, Monograph Ser. Fla agric. Exp. Stn 4, 398 pp., 1974a.
- Edwardson, Monograph Ser. Fla agric. Exp. Stn 5, 225 pp., 1974b.
- Fraenkel-Conrat, in Comprehensive Virology Vol. 1, ed. H. Fraenkel-Conrat & R. R. Wagner, 191 pp., New York: Plenum Press, 1974.
- Govier & Kassanis, Virology 61: 420, 1974.
- Govier & Woods, J. gen. Virol. 13: 127, 1971.
- Gugerli & Gehringer, Potato Res. 23: 353, 1980.
- Hiebert & McDonald, Virology 56: 349, 1973.
- Hinostroza-Orihuela, Virology 67: 276, 1975.
- Horváth, Acta phytopath. Acad. Sci. hung. 14: 157, 1979.
- Huttinga, Neth. J. Pl. Path. 79: 125, 1973.
- Huttinga, Neth. J. Pl. Path. 81: 58, 1975.
- Huttinga & Mosch, Neth. J. Pl. Path. 80: 19, 1974.
- Kennedy, Day & Eastop, A Conspectus of Aphids as Vectors of Plant Viruses, 114 pp., London: Commonwealth Institute of Entomology, 1962.
- Kitajima, Camargo & Costa, J. Electr. Microsc. 17: 144, 1968.
- Klinkowski & Schmelzer, Am. Potato J. 37: 221, 1960.
- Leiser & Richter, Arch. Phytopath. PflSchutz. 14: 337, 1978.
- Leiser & Richter, Arch. Phytopath. PflSchutz. 15: 289, 1979.
- Loebenstein & Raccah, Phytoparasitica 8: 221, 1980.
- Makkouk & Gumpf, Phytopathology 64: 1115, 1974.
- Makkouk & Gumpf, Virology 63: 336, 1975.
- McDonald, Beveridge & Bancroft, Virology 69: 327, 1976.
- Miki & Oshima, J. gen. Virol. 15: 179, 1972.
- Moghal & Francki, Virology 73: 350, 1976.
- Purcifull, CMI/AAB Descriptions of Plant Viruses 161, 4 pp., 1976.
- Purcifull & Batchelor, Bull. Fla agric. Exp. Stn 788, 39 pp., 1977.
- Purcifull & Gooding, Phytopathology 60: 1036, 1970.
- Purcifull, Hiebert & McDonald, Virology 55: 275, 1973.
- Rubio-Huertos, in Principles and Techniques in Plant Virology, ed. C. J. Kado & H. O. Agrawal, 300 pp., New York: Van Nostrand Reinhold Company, 1972.
- Schmelzer, Bartels & Klinkowski, Phytopath. Z. 40: 52, 1960.
- Sievert, Phytopathology 68: 974, 1978.
- Smith, Proc. R. Soc. Lond. B 109: 251, 1931.
- Stace-Smith & Tremaine, Phytopathology 60: 1785, 1970.
- Thresh, Appl. Biol. 5: 1, 1980.
- Todd, Proc. 4th Conf Potato Virus Diseases, Braunschweig: 82, 1961.
- Vanderveken, in Aphids as Virus Vectors, ed. K. F. Harris & K. Maramorosch, 559 pp., New York: Academic Press, 1977.
- Van Hoof, Neth. J. Pl. Path. 86: 159, 1980.
- Varma, Gibbs, Woods & Finch, J. gen. Virol. 2: 107, 1968.
- Watson, Ann. appl. Biol. 44: 599, 1956.
- Watson & Roberts, Proc. R. Soc. Lond. B. 127: 543, 1939.