Details of DPV and References
DPV NO: 246 July 1982
Family: Secoviridae
Genus: Comovirus
Species: Bean rugose mosaic virus | Acronym: BRMV
Bean rugose mosaic virus
R. Gámez Centro de Investigación en Biologia Celular y Molecular, Universidad de Costa Rica, Ciudad Universitaria, Costa Rica
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by
Gámez (1972).
A virus with isometric particles c. 28 nm in diameter sedimenting as three components, two of which contain single-stranded RNA. Transmitted by beetles and readily by inoculation of sap. Narrow host range, mainly restricted to legumes. Found in Costa Rica and other countries in Central America.
Main Diseases
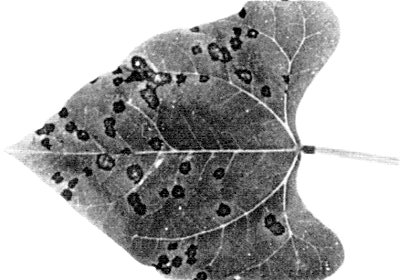
Causes a severe mosaic, rugosities and deformation of leaves (Fig. 1) of bean (Phaseolus vulgaris). Mottling and malformation of pods, and stunting are observed in susceptible varieties (Gámez, 1972; 1980).
Geographical Distribution
Reported from Costa Rica (Gámez, 1972), El Salvador (Gálvez et al., 1977) and Guatemala (Cartín, 1973).
Host Range and Symptomatology
Occurs naturally in bean (Phaseolus vulgaris). Restricted host range: only species of Leguminosae are systemically infected following mechanical inoculation, and Chenopodium amaranticolor is the only local lesion host found among 15 species in 5 dicotyledonous families (Gámez, 1972).
-
Diagnostic species
- Phaseolus vulgaris.
Three different types of reaction, depending on cultivar. In some (e.g. cv. Plentiful) there is systemic infection resulting in severe mosaic, rugosity, blistering and deformation of leaves (Fig. 1, Fig. 2) and mottling and malformation of pods; others (e.g. cv. Top Crop) develop necrotic lesions in inoculated leaves (Fig. 3) but no systemic infection. Cv. Tendergreen is immune. - Chenopodium amaranticolor. Local necrotic lesions. No systemic
infection.
- Cucumis sativus. Immune.
- Nicotiana tabacum cv. White Burley. Immune.
- Vicia faba. Mild mottle in systemically infected leaves.
- Cucumis sativus. Immune.
-
Propagation species
- Phaseolus vulgaris
cvs Plentiful, Col. 109-R and Méx. 27-R.Assay species
- Phaseolus vulgaris
cvs Top Crop, Pinto III, Jamapa.
Strains
Three strains have been recognized; a mild and a severe strain from Guatemala (Cartín, 1973; Kitajima et al., 1974), and the severe ‘ampollado’ strain from El Salvador (Gálvez et al., 1977). Strains differ in host range, cytopathology and symptomatology, but appear to be serologically identical or closely related.
Transmission by Vectors
Transmitted by the chrysomelid beetles Cerotoma ruficornis, Diabrotica balteata and D. adelpha. Insects acquire the virus in 12 to 24 h but differ in the length of time for which they remain viruliferous; C. ruficornis retains the virus for 9 to 10 days and D. balteata for 3 to 4 days (Fulton, Scott & Gámez, 1980; Gámez, 1972). Frequency of transmission on the first day is much greater for C. ruficornis (80%) than for D. balteata (20%) or D. adeipha (10%). Beetles may be rendered viruliferous by intrahaemocoelic injection. The virus may be recovered from faeces, regurgitated fluid and haemolymph of C. ruficornis.
Transmission through Seed
Not seed-transmitted in Phaseolus vulgaris (Gámez, 1972).
Serology
The virus is very immunogenic. The ring precipitin and gel double diffusion tests are useful. Rabbit antisera with titres of 1/1024 in the ring precipitin test are easily obtained (Gámez, 1972). Serologically specific electron microscopy has been used for the identification of the virus.
Relationships
Strains from Guatemala and El Salvador are serologically closely related to the type strain from Costa Rica (Cartín, 1973; Fulton & Scott, 1979; Gálvez et al., 1977). Bean rugose mosaic virus is a comovirus, distantly related to bean pod mottle, cowpea severe mosaic and cowpea mosaic viruses (Gámez, 1972). Antiserum to bean rugose mosaic virus reacts with other comoviruses to give diffuse straight bands of precipitate which, following the criteria of Fulton & Scott (1977; 1979), suggests that it should be regarded as a distinct member of the comovirus group.
Stability in Sap
In extracts of P. vulgaris cv. Col. 109-R assayed in cv. Pinto III, the thermal inactivation point (10 min) is between 65 and 70°C, the longevity in vitro is between 2 and 4 days at 22°C, and the dilution end-point is between 10-4 and 10-5 (Gámez, 1972).
Purification
Steere’s chloroform/butanol method, as modified by Bancroft (1962) for the purification of bean pod mottle virus, is suitable. Extract infected P. vulgaris leaves in 3.5 ml 50% K2HPO4/100 g of tissue, express the juice and extract the pulp in 0.01 M phosphate buffer, pH 7.0. Combine the extracts and clarify with a 1:1 mixture of chloroform and n-butanol, followed by one cycle of differential centrifugation. Precipitate the virus at pH 5.0 with 10% acetic acid. Resuspend in 0.5 M phosphate buffer, pH 7.0, and give a further cycle of differential centrifugation. An alternative method is clarification with butanol and chloroform, and precipitation by adding polyethylene glycol (M. Wt 6000) to 4% and NaCl to 0.2 M. The precipitate is resuspended, clarified by centrifugation at low speed, and sedimented through a sucrose cushion, as described for cowpea mosaic virus (van Kammen & de Jager, 1978).
Properties of Particles
The virus sediments as three components: empty protein shells (T), and two nucleoproteins with different RNA content (M and B) (Gámez, 1972).
Sedimentation coefficients (s20,w ) (svedbergs) are: 59
(T), 97 (M), 113 (B) (H. A. Scott, unpublished data).
A260/A280: 1.7 for unfractionated virus, uncorrected for light- scattering (Gámez, 1972).
Buoyant densities in caesium chloride: 1.302 (T), 1.380 (M) and 1.402 (B) g/cm3 (Lucía Fuentes & R. Gámez, unpublished data).
Molecular weight (x 10-6): about 3.6 (T), 5.0 (M) and 5.9 (B), estimated from the protein and RNA composition.
Particle Structure
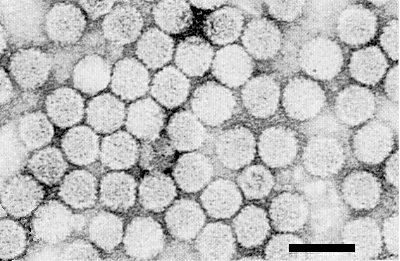
Particles are isometric, c. 28-29 nm in diameter with hexagonal outlines (Fig. 4).
Particle Composition
Nucleic acid: RNA, single-stranded, of M. Wt about 2.3 x 106 and 1.4 x 106, estimated by polyacrylamide gel electrophoresis under denaturing conditions (Lucía Fuentes & P. Leon, unpublished data).
Protein: Polyacrylamide gel electrophoresis of SDS-treated virus shows two polypeptide species of M. Wt c. 38,000 and 22,000. By analogy with other comoviruses, there are presumably 60 molecules of each polypeptide per particle.
Relations with Cells and Tissues
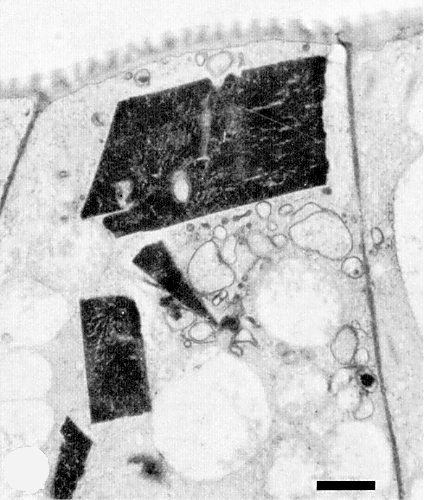
Trichomal, epidermal and mesophyll cells of infected P. vulgaris leaves show large, dense, crystalline inclusions in the cytoplasm. These crystals are made up of virus particles, many of which are in hexagonal arrays (Fig. 5, Fig. 6). A less dense crystalline material, exhibiting a honeycomb-like pattern, is occasionally observed. Particles also occur scattered in the cytoplasm and in tubules in the plasmodesmata, associated with finger-like outgrowths of the cell wall. The cytoplasm around virus crystals frequently appears rich in vesicles and tubules (Fig. 6). Strains differ in the type of cellular alteration that is most prevalent (Kitajima et al., 1974).
Notes
Among comoviruses, cowpea severe mosaic virus and bean pod mottle virus are the most closely related to bean rugose mosaic virus (Bruening, 1978; Gámez, 1972), but may be distinguished by differences in host range and symptomatology in indicator hosts and by serological reactions. Vigna unguiculata cv. Early Ramshorn is readily infected by cowpea severe mosaic virus but is not infected by bean rugose mosaic virus. P. vulgaris cv. Tendergreen is susceptible to cowpea severe mosaic and bean pod mottle viruses but not to bean rugose mosaic virus. Antigenic specificity, disease symptoms and type of insect vector readily distinguished this virus from other viruses occurring naturally in Phaseolus bean (Fulton & Scott, 1977; 1979; Gámez, 1972). Bean rugose mosaic virus is of limited economic importance because most bean cultivars grown in Central America are resistant (Gámez, 1972; 1980).
Figures
References list for DPV: Bean rugose mosaic virus (246)
- Bancroft, Virology 16: 419, 1962.
- Bruening, CMI/AAB Descr. Pl. Viruses 199, 5 pp., 1978.
- Cartín, Ing. Agron. Thesis, Univ. of Costa Rica, 1973.
- Fulton & Scott, Fitopatol. Bras. 2: 9, 1977.
- Fulton & Scott, Phytopathology 69: 305, 1979.
- Fulton, Scott & Gámez, in Vectors of Plant Pathogens,p. 115, ed. K. Maramorosch & K. Harris, New York: Academic Press, 467pp., 1980.
- Gálvez, Cárdenas, Kitajima, Diaz & Nieto,Turrialba 27: 343, 1977.
- Gámez, Turrialba 22: 249, 1972.
- Gámez, in Problems in Bean Production, p. 241, ed. H. F.Schwartz & G. E. Gálvez, Cali, Colombia: CIAT, 424 pp., 1980.
- Kitajima, Tascón, Gámez & Gálvez, Turrialba 24: 393, 1974.
- van Kammen & de Jager, CMI/AAB Descr. Pl. Viruses 197, 6 pp., 1978.