Details of DPV and References
DPV NO: 257 July 1982
Family: Luteoviridae
Genus: Enamovirus
Species: Pea enation mosaic virus | Acronym: PEMV
There is a more recent description of this virus: DPV 372. RNA1 is now PEMV-1 (Genus Enamovirus); RNA2 is PEMV-2 (Genus Umbravirus)
This is a revised version of DPV 25
Pea enation mosaic virus
D. Peters Agricultural University, Department of Virology, Binnenhaven 11, 6709 PD Wageningen, The Netherlands
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by
Osborn (1935).
Selected synonyms
-
Pea virus 1 (Rev. appl. Mycol. 15: 418)
- Pisum virus 1 (Rev. appl. Mycol. 17: 52)
-
A virus with isometric particles of two kinds, both about 28 nm in diameter, but each containing one of the two species of ssRNA that comprise the genome. The virus is transmitted by aphids in a persistent manner and readily by sap inoculation. It has a restricted host range, mostly in leguminous plants, and is common in northern temperate regions.
Main Diseases
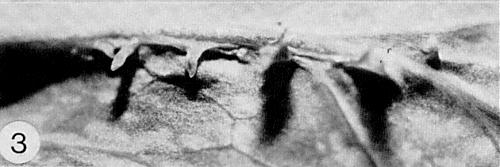
Causes common diseases of sweet pea (Lathyrus odoratus), garden and field pea (Pisum sativum), and broad bean (Vicia faba). Infected plants of these species develop mosaic, hyaline spots and, eventually, enations (Fig. 1, Fig. 2, Fig. 3). In Oregon, USA, alfalfa (Medicago sativa) is probably the principal local source of the virus, and subterranean clover (Trifolium subterraneum) is an important natural host (McWhorter & Cook, 1958). Yield losses of up to about 50% have been reported in V. faba (Heathcote & Gibbs, 1962).
Geographical Distribution
Widely distributed in northern temperate regions, but also reported in warmer regions such as Sicily (Rosciglione & Cannizzaro, 1975) and Iran (Kaiser, Mossahebi & Okhovat, 1971). Schroeder & Barton (1958) considered the virus to be one of the most important causes of disease in pea in New York State, USA.
Host Range and Symptomatology
Most of the reported host ranges are similar. The virus infects many legumes (Thottappilly, 1972; Hull, 1981) but only a few species in other families. Most if not all leguminous species show mosaic, enations and often malformation of leaves and plants; the reactions are more severe on younger than on older plants. A slight vein-clearing can be found on the leaves of P. sativum and V. faba about 6 or 7 days after infection. The virus induces local lesions in Chenopodium album, C. amaranticolor and C. quinoa, and mosaic and malformation in Nicotiana clevelandii (Hagedorn, Layne & Ruppel, 1964; Gibbs, Harrison & Woods, 1966). Susceptibility of N. tabacum cv. White Burley has been reported by Motoyoshi & Hull (1974). The severity of symptoms can vary with pea cultivar and with the isolate of virus. Resistance has been reported in pea (Schroeder & Barton, 1958). Large differences in resistance were found by Hagedorn & Hampton (1975) in 43 pea lines.
-
Diagnostic species
- Pisum sativum
(garden pea). Slight vein-clearing occurs on the young leaves about 6 to 8 days after inoculation. This is followed by chlorotic flecks, hyaline and translucent spots on the leaves, and later by mosaic patterns and upward rolling of young leaves. Plants inoculated when young show severe distortion and stunting within 10 days (Fig. 1). Chronically infected plants show vein-clearing or mosaic (Fig. 2) and usually develop enations (Fig. 3) associated with the veins on the undersides of the upper leaves (Hagedorn et al., 1964; Quantz, 1952). -
Trifolium incarnatum and Vicia faba. Symptoms in these species are similar
to those in P. sativum.
Propagation species
- Pisum sativum.
In plants grown at 18-22°C, virus concentrations are greatest 10-14 days after mechanical inoculation and fall rapidly thereafter (Izadpanah & Shepherd, 1966). -
Assay species
- Chenopodium album, C. amaranticolor
and C. quinoa can be used for local lesion assay, though not all seed stocks of these species give suitable local lesions. Hull & Lane (1973) considered C. quinoa the most reliable, whereas Izadpanah & Shepherd (1966) preferred C. album. Pisum sativum seedlings are the most frequently used test plants in aphid-transmission studies.
Strains
The majority of isolates used in various studies are field isolates. They may differ in symptomatology, ease of mechanical transmission, aphid transmissibility (Osborn, 1938; Bath & Chapman, 1967), yield after purification, and relative amounts of top and bottom component (Hull & Lane, 1973; Hull, 1977b). Four groups were recognised on the basis of electrophoretic behaviour (Hull, 1977b). On repeated transfers isolates may lose their transmissibility by aphids (Clarke & Bath, 1977), and this is associated with loss of one of the particle proteins and of a segment of RNA-1 (see Particle Composition).
Transmission by Vectors
Transmitted in a circulative way by at least seven aphid species, Acyrthosiphon pisum being the most efficient vector in the field. Transmission of the virus from pea to pea plants has been obtained with three grain aphid species for which pea is a non-host (Nault, 1975). Transmission efficiency varies with the strain of A. pisum (Tsai, Bath & Igbokwe, 1972). Strains of the virus exist that are not transmissible by A. pisum but the aphid could transmit a non-transmissible strain from plants that were also infected by a transmissible strain (Adam, 1978).
All instars of A. pisum transmit, nymphs more efficiently than adults. Nymphs can acquire virus in 15 min, adults in 1-2 h; all instars exhibit a temperature-dependent latent period (Simons, 1954; Bath & Chapman, 1968). Nymphs allowed an acquisition access period of 3 h and then transferred hourly at 20°C, exhibited a latent period (as estimated by the method of Sylvester, 1965) of approximately 10 h (Toros, Schotman & Peters, 1978). After the latent period, A. pisum can inoculate plants in brief test probes, which shows that the virus can infect plants following aphid inoculation of superficial tissues. The longer the inoculation feed, the greater the proportion of plants infected (Nault & Gyrisco, 1966). If an aphid reaches the end of its latent period while feeding on a healthy test plant, the virus may not be transmitted unless the stylets are withdrawn and feeding is re-initiated (Toros et al., 1978). The length of the latent period increases and the frequency of transmission declines with the age of the aphid; ‘recharging’ of the aphid restores the transmission efficiency only partly (Sylvester & Richardson, 1966).
The virus is retained after moulting. When haemolymph from aphids that had acquired the virus from plants was injected into virus-free aphids, the recipients became able to transmit the virus, but a second set of recipients injected with haemolymph from the first set did not transmit (Clarke & Bath, 1973). There is no evidence for virus replication in the aphid, but virus particles can be found in vector cells (see Relationships with Cells and Tissues). Aphids can acquire the virus from purified preparations through Parafilm membranes (Thottappilly, Bath & French, 1972), and become infective after injection with honeydew (Richardson & Sylvester, 1965). Virus can be detected in individual aphids by enzyme-linked immunosorbent assay (ELISA) (Fargette, Jenniskens & Peters, 1982); the amount acquired by individual aphids varies considerably and is poorly correlated with the length of the latent period and with the ability of the aphids to transmit. Most of the virus acquired accumulates in the intestinal tract and is flushed out by feeding on healthy plants; only small amounts are detected in the haemocoele (Fargette et al., 1982).
No significant decrease of transmission is caused by applying mineral oil to leaves (Peters & Lebbink, 1973).
Transmission through Seed
Blattny (1956) reported a low frequency (1.5%) of seed transmission in Pisum sativum. Kovachevski (1978) also mentioned seed transmission.
Serology
The virus is moderately immunogenic. Standard methods using rabbits give antisera with titres of 1/128 in the Ouchterlony double diffusion test. Sap from infected plants contains a soluble antigen (Izadpanah & Shepherd, 1966). The soluble antigen was detected only in plants infected with aphid-transmissible isolates and not in plants infected with non-transmissible isolates; antisera to aphid-transmissible isolates contained two antibody populations but those produced against non-transmissible isolates contained only one antibody population (Clarke & Bath, 1977). The ELISA technique can detect the virus in plants and aphids in concentrations down to 5 ng/ml (Fargette et al., 1982).
Relationships
No serologically related viruses have been reported.
Stability in Sap
Various values have been reported. Osborn (1938) gives a thermal inactivation temperature of about 65°C, a dilution end-point of 10-4 and longevity of 4 days at 20°C in sap of Vicia faba, whereas Pierce (1935) reports a thermal inactivation point of 56 to 58°C and a dilution end-point of 10-3 in sap of Pisum sativum. Infectivity in sap seems to be retained best at pH 6.0.
Purification
Good results have been obtained with various methods (Izadpanah & Shepherd, 1966; Bozarth & Chow, 1966; Gibbs et al., 1966; Mahmood & Peters, 1973) and may depend on the isolate used. The method of Mahmood & Peters (1973), which seems generally useful, is as follows. Homogenize each 1 g infected tissue in 2 ml 0.15 M acetate buffer, pH 6.1, and 1 ml chloroform. Strain the emulsion through cheesecloth and centrifuge the filtrate at 10,000 rev./min for 15 min. Adjust the pH of the supernatant fluid to 5.3 and remove the flocculated material by centrifugation. Readjust the pH of the supernatant fluid to pH 6.1 and concentrate the virus from it by high speed centrifugation or by adding polyethylene glycol, M. Wt 6000, to 6% (w/v), and centrifuging at low speed. Resuspend the pellets in 0.1 M acetate buffer. The virus particles may be further purified by centrifugation in sucrose density gradients. Incorporation of 0.015 M MgCl2 into the acetate buffer may stabilize infectivity (Mahmood & Peters, 1973). Resuspension of pellets in buffer plus 5% sucrose may increase virus yield (Bozarth & Chow, 1968).
Properties of Particles
Purified preparations of virus particles consist of two sedimenting components, top (T) and bottom (B), containing RNA (Fig. 5); RNA-free particles have not been found. The separated components are not infective (Hull & Lane, 1973), but mixtures of the two components are. Mixtures of one component of the wild type and the other from a variant type are also infective (Hull & Lane, 1973). The relative amount of the components depends on the virus isolate but B component usually predominates. A variant strain isolated by Hull & Lane (1973) consisted mainly of T component. T component degrades more rapidly than B component at high salt concentration and at high pH (Hull, 1977b).
Sedimentation coefficients (s20,w) at infinite dilution (svedbergs): 112 (B) and 99 (T).
Particle weight: 5.57 x 106 (B) and 4.38 x 106 (T), calculated from the Svedberg equation, or 5.71 x 106 (B) and 4.64 x 106 (T), estimated from the nucleic acid weight and content (Hull & Lane, 1973).
Isoelectric point: Between pH 5 and 6 (Izadpanah & Shepherd, 1966); pH 5.9 (Hull, 1977b). These values seem to be incompatible with the buffer pH used in the purification of the virus. Precipitation at values above 7 may be caused by distortion of the particles (Hull, 1976).
Buoyant density (g/cm3): 1.36 in D2O (both components) (Hull & Lane, 1973); 1.380 in Cs2SO4 (both components) (Hull, 1976); and 1.436 in CsCl (B component; the top component degrades in CsCl) (Hull & Lane, 1973).
A260 (1 mg/ml, 1 cm light path): 7.2 (unfractionated virus). No differences have been found between the top and bottom component.
A260/A280: 1.63 (unfractionated virus).
Electrophoretic components: Purified preparations of isolates that are not aphid-transmissible contain two electrophoretic components, the slower-migrating one corresponding to B component and the faster one to T component (Hull & Lane, 1973). In contrast, both sedimenting components of aphid-transmissible isolates give rise to multiple electrophoretic components (Adam, Sander & Shepherd, 1979) and this phenomenon is linked with the presence of an additional protein in the particles of these isolates (see Particle Structure and Particle Composition).
Particle Structure
Most particles in purified preparations are isometric, and have a diameter of c. 26 nm (Fig. 4). Most particles appear hexagonal in outline; however, a few are pentagonal and others are more ovoid, and such particles are found more frequently in fractions rich in T component than in those rich in B component. Minute projections are seen on some particles (Bozarth & Chow, 1966). Hull & Lane (1973) suggested that the B component contained 180 of the 22,000 M. Wt subunits and the T component 140-150, each pair of subunits in a hexamer on the twofold axes being replaced by a single subunit. A trimer clustering instead of pentamer-hexamer clustering was suggested by Musil, Marcinka & Ciampor (1970). Addition of the 56,000 dalton protein to the particles of aphid-transmissible isolates probably occurs stepwise, resulting in incremental increases of particle size (Adam et al., 1979) which would account for the multiple electrophoretic bands observed in these isolates.
Particle Composition
Nucleic acid: RNA, single-stranded; 28% by weight of particle. Two genomic RNA species are detected: M. Wt estimates for RNA-1, found in component B, range from 2.3 to 1.4 x 106 and those for RNA-2, found in component T, range from 1.68 to 1.1 x 106 (Hull, 1981). A smaller RNA component of M. Wt c. 2 x 105 (RNA-3) is often found and may arise by cleavage of RNA-1 to give RNA-3 and a species of the same size as RNA-2 (Adam et al., 1979; German & de Zoeten, 1975). The base composition (molar percentage of nucleotides) for the total RNA is G 26.6; A 24.1; C 24.5; U 24.8 (Mahmood & Peters, 1973). The RNA molecules have no poly(A) tails and cannot be aminoacylated (German, de Zoeten & Hall, 1978). Their 5' ends are not capped, but are covalently bound to a genome-linked protein (Reisman & de Zoeten, 1982). RNA-1 codes for the 22,000 M. Wt coat protein (Hull & Lane, 1973). RNA-1 may also carry the genetic information for the 56,000 M. Wt protein because the RNA-1 molecule of non-aphid-transmissible strains is smaller than that of aphid-transmissible strains and may lack the portion of the molecule that codes for the 56,000 M. Wt particle protein (Adam et al., 1979). Preparations of double-stranded RNA isolated from infected plants contain molecules that correspond to each of the genome RNA species (German & de Zoeten, 1975).
Protein: Particles of all isolates contain a major protein of M. Wt c. 22,000, estimated by electrophoresis in polyacrylamide/SDS gels; a component presumed to be a dimer, of M. Wt c. 44,000, is found in some preparations (Hull & Lane, 1973). The 22,000 M. Wt protein consists of 199 amino-acid residues (Shepherd, Wakeman & Ghabrial, 1968). In addition, particles of aphid-transmissible isolates contain a second protein, in considerably less than equimolar amounts, with M. Wt estimated at either 28,000 (Hull, 1977a) or 56,000 (Adam et al., 1979). This protein is thought to mediate transmission of the virus by aphids. The genome-linked protein has an estimated M. Wt of 17,500 (Reisman & de Zoeten, 1982).
Relations with Cells and Tissues
No inclusion bodies are found. Virus particles occur both within the nuclei and in the cytoplasm, either scattered or in crystalline aggregates (Shikata & Maramorosch, 1966; de Zoeten, Gaard & Diez, 1972). In early stages of infection the virus is found only in the nuclei, where synthesis of virus-specific RNA occurs (Fig. 6) (de Zoeten et al., 1976). In later stages of infection the nucleoli and parts of the nuclei of infected cells are destroyed and the particles escape into the cytoplasm (Shikata & Maramorosch, 1966). The affected nuclei are devoid of chromatin. Vesicles containing fibrillar material (Fig. 7) occur in the perinuclear space and originate from the inner nuclear membrane; groups of these vesicles occur throughout the cytoplasm, still encapsulated in a membrane which is probably derived from the outer membrane of the nuclear envelope (de Zoeten, Gaard & Diez, 1972). RNA-dependent RNA polymerase activity is associated with these structures and they contain virus specific ds-RNA (Powell & de Zoeten, 1977). Structures with a high electron opacity protrude from the plasmodesmata into the cytoplasm of infected and apparently healthy companion cells and sieve elements of infected plants. These structures which are found between the 4th and 7th day after inoculation are thought to play a role in the systemic invasion of the plant by the virus (G. A. de Zoeten & G. Gaard, personal communication).
Particles are not found in mitochondria or chloroplasts; these organelles are fewer and smaller in infected cells than in healthy ones (Shikata & Maramorosch, 1966).
The virus can infect tobacco protoplasts, and infection can occur in the absence of poly-L-ornithine. Actinomycin D inhibits the accumulation of virus antigen in protoplasts and this effect was not restricted to either early or late phases of infection (Motoyoshi & Hull, 1974).
Numerous particles are found in the lumen of the midgut of Acyrthosiphon pisum vector aphids; midgut cells may contain few or no particles or large accumulations. Particles occur in fat-body cells but rarely in muscle cells and haemocytes; none have been found in the other tissues (Harris & Bath, 1972). Particles have been found only rarely in the nuclei of the midgut cells. There is no evidence that the presence of virus particles in these cells and nuclei results from replication. Accumulation of antigen in cells of primary aphid cell cultures has been reported (Adam & Sander, 1976), but it is not clear whether this can be considered evidence for virus multiplication in these cells.
Notes
The virus is readily distinguished from all others by its characteristic symptoms, its mechanical transmissibility, its persistence in its aphid vector and its sedimentation profile. The virus is not serologically related to any of the known plant viruses.
Figures

(Left) Pea (Pisum sativum), 3 weeks old, infected 6 days after sowing; (right) healthy plant of the same age.

Portions of systemically infected (left and centre) or healthy (right) plants of P. sativum. (Courtesy R. J. Shepherd.)
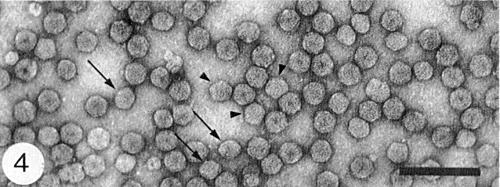
Virus particles from a purified preparation mounted in uranyl acetate. Short arrow: particles with pentagonal outlines; long arrow: particles with ovoid outlines. Bar represents 100 nm.
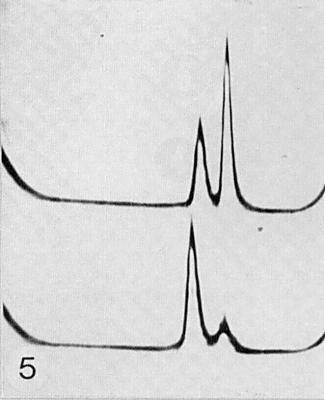
Sedimentation patterns of purified preparations: (above) a wild type strain, (below) a variant (Hull & Lane, 1973). Sedimentation from left to right. (Courtesy R. Hull.)
References list for DPV: Pea enation mosaic virus (257)
- Adam, Z. PflKrankh. PflPath. PflSchutz 85: 586, 1978.
- Adam & Sander, Virology 70: 502, 1976.
- Adam, Sander & Shepherd, Virology 92: 1, 1979.
- Bath & Chapman, Virology 33: 503, 1967.
- Bath & Chapman, Ann. ent. Soc. Am. 62: 907, 1968.
- Blattny, Trudy Inst. Genet. 23: 317, 1956.
- Bozarth & Chow, Contr. Boyce Thompson Inst. Pl. Res. 23: 301, 1966.
- Bozarth & Chow, Virology 36: 506, 1968.
- Clarke & Bath, Ann. ent. Soc. Am. 66: 603, 1973.
- Clarke & Bath, Phytopathology 67: 1035, 1977.
- de Zoeten, Gaard & Diez, Virology 48: 638, 1972.
- de Zoeten, Powell, Gaard & German, Virology 70: 459, 1976.
- Fargette, Jenniskens & Peters, Phytopathology 72: 1386, 1982.
- German & de Zoeten, Virology 66: 172, 1975.
- German, de Zoeten & Hall, Intervirology 9: 226, 1978.
- Gibbs, Harrison & Woods, Virology 29: 348, 1966.
- Hagedorn & Hampton, Pl. Dis. Reptr 59: 895, 1975.
- Hagedorn, Layne & Ruppel, Phytopathology 54: 843, 1964.
- Harris & Bath, Virology 50: 778, 1972.
- Heathcote & Gibbs, Pl. Path. 11: 69, 1962.
- Hull, Virology 75: 18, 1976.
- Hull, J. gen. Virol. 34: 183, 1977a.
- Hull, in Aphids as Virus Vectors, p. 137, ed. K. F. Harris & K. Maramorosch, New York:Academic Press, 559 pp., 1977b.
- Hull, in Handbook of Plant Virus Infections and Comparative Diagnosis, p.239, ed. E.Kurstak, Amsterdam: Elsevier/North-Holland, 943 pp., 1981.
- Hull & Lane, Virology 55: 1, 1973.
- Izadpanah & Shepherd, Virology 28: 463, 1966.
- Kaiser, Mossahebi & Okhovat, Iranian J. Pl. Path. 7: 25, 1971.
- Kovachevski, Rasteniev"dni Nauki 15: 108, 1978.
- Mahmood & Peters, Neth, J. Pl. Path. 79: 138, 1973.
- McWhorter & Cook, Pl. Dis. Reptr 42: 51, 1958.
- Motoyoshi & Hull, J. gen. Virol. 24: 89, 1974.
- Musil, Marcinka & Ciampor, Acta virol., Prague 14: 285, 1970.
- Nault, Phytopathology 65: 496, 1975.
- Nault & Gyrisco, Ann. ent. Soc. Am. 59: 1185, 1966.
- Osborn, Phytopathology 25: 160, 1935.
- Osborn, Phytopathology 28: 923, 1938.
- Peters & Lebbink, Entomologia exp. appl. 16: 185, 1973.
- Pierce, J. agric. Res. 51: 1017, 1935.
- Powell & de Zoeten, Virology 78: 135, 1977.
- Quantz, NachrBl. dt. PflSchutzdienst (Braunschw.) Stuttg. 4: 24, 1952.
- Reisman & de Zoeten, J. gen. Virol., 62: 187, 1982.
- Richardson & Sylvester, Virology 25: 472, 1965.
- Rosciglione & Cannizzaro, Phytopath. Medit. 14: 34, 1975.
- Schroeder & Barton, Phytopathology 48: 628, 1958.
- Shepherd, Wakeman & Ghabrial, Virology 35: 255, 1968.
- Shikata & Maramorosch, Virology 30: 439, 1966.
- Simons, Phytopathology 44: 283, 1954.
- Sylvester, Virology 25: 62, 1965.
- Sylvester & Richardson, Virology 30: 592, 1966.
- Thottappilly, Z. PflKrankh. PflPath. PflSchutz 79: 686, 1972.
- Thottappilly, Bath & French, Virology 50: 681, 1972.
- Toros, Schotman & Peters, Virology 90: 235, 1978.
- Tsai, Bath & Igbokwe, Ann. ent. Soc. Am. 65: 1114, 1972.