Details of DPV and References
DPV NO: 270 July 1983
Family: Secoviridae
Genus: Cheravirus
Species: Arracacha virus B | Acronym: AVB
Arracacha virus B
R. A. C. Jones MAFF, Harpenden Laboratory, Hatching Green, Harpenden, Hertfordshire, UK
R. H. Kenten Lately, Rothamsted Experimental Station, Harpenden, Hertfordshire, UK
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
Described by Kenten & Jones (1979).
A virus with c. 26 nm diameter isometric particles that sediment as a single component and contain polypeptides that migrate as two components in SDS/polyacrylamide gel electrophoresis. The virus is readily sap-transmissible and has a wide experimental host range but has been found occurring naturally only in arracacha, oca and potato. The virus is transmitted through seed but its vector is unknown. Two very distinct strains occur. Found in the Andean region of South America.
Main Diseases
The virus is found naturally in arracacha (Arracacia xanthorrhiza; Umbelliferae), oca (Oxalis tuberosa; Oxalidaceae) and potato (Solanum tuberosum; Solanaceae). The virus causes symptomless infection in experimentally infected potato plants, but attempts to return it to healthy arracacha and oca were unsuccessful. Naturally infected plants of arracacha and oca growing in the glasshouse showed no symptoms. Whether the virus sometimes causes symptoms is unknown: it has always been present in mixed infection with other viruses when found in plants with symptoms in the field (Kenten & Jones, 1979; Jones & Kenten, 1981; Jones, 1981; Atkey & Brunt, 1982).
Geographical Distribution
Reported from Peru and Bolivia.
Host Range and Symptomatology
The virus is readily transmissible by inoculation of sap and has a wide experimental host range, infecting species in at least eight dicotyledonous families (Kenten & Jones, 1979; Jones & Kenten, 1981). The oca strain (strain O) and the type strain (strain T) differ in their effects on most hosts; strain O is the milder of the two.
- Diagnostic species
- Chenopodium amaranticolor: Small chlorotic or necrotic spots or rings
in inoculated leaves; systemic symptoms are a mild mosaic with strain O or a
systemic chlorotic mottle (Fig. 1) with down-curling of leaves with strain T.
With both strains, leaves produced later are symptomless (‘recovery’).
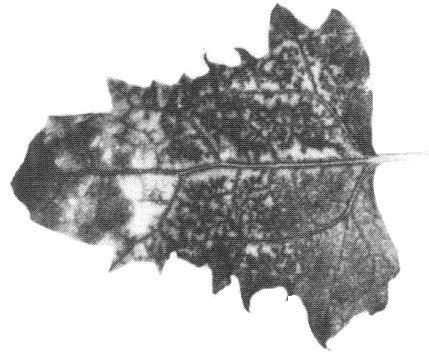
- C. murale: Occasional expanding necrotic spots and/or ringspots in inoculated leaves; systemic chlorotic mottle and twisting of young leaves followed by necrosis of the tip and upper leaves (Fig. 2).
- Cucumis sativus: Systemic mild mosaic; leaves produced later are symptomless (‘recovery’).
- C. murale: Occasional expanding necrotic spots and/or ringspots in inoculated leaves; systemic chlorotic mottle and twisting of young leaves followed by necrosis of the tip and upper leaves (Fig. 2).
- Propagation species:
- Chenopodium quinoa: Strain O causes symtomless systemic infection
or a faint mosaic. Strain T induces chlorotic spots, rings or ringspots in
inoculated leaves (Fig. 3) followed by systemic mottle (Fig. 4) with slight
distortion or down-curling of the leaves. With both strains, leaves produced
later are symptomless (‘recovery’).
- Tetragonia expansa: Strain T induces systemic mottle or mosaic followed by deformation and twisting of leaves. Strain O causes similar but milder symptoms.
- Tetragonia expansa: Strain T induces systemic mottle or mosaic followed by deformation and twisting of leaves. Strain O causes similar but milder symptoms.
- Assay species:
- Chenopodium quinoa can be used as a local lesion host for strain T and C. amaranticolor for both strains. C. amaranticolor and C. murale are suitable for whole plant assays.
Strains
Strain T from arracacha (Kenten & Jones, 1979) and strain O from oca and potato (Jones, 1981; Jones & Kenten, 1981) are serologically only distantly related. Only strain O infects potato, whereas only strain T infects Cyamopsis tetragonoloba and Vigna unguiculata ssp. sinensis systemically. Chenopodium murale and cucumber are the only hosts known that develop identical symptoms with both strains.
Transmission by Vectors
No vector is known. Neither strain is transmitted by Myzus persicae (Kenten & Jones, 1979; Jones & Kenten, 1981).
Transmission through Seed
Both strains are readily transmitted through seed of C. quinoa (Kenten & Jones, 1979; Jones & Kenten, 1981) and strain O through true seed of potato (Solanum tuberosum). Strain O is pollen-transmitted to seed produced on a healthy potato plant but not to the plant itself (Jones, 1982).
Serology
Antisera with titres of 1/512 (strain T) and 1/256 (strain O) in gel-diffusion precipitin tests were readily obtained. The virus produces a single band of precipitate in double diffusion tests in agar gel (Kenten & Jones, 1979; Jones & Kenten, 1981). Both gel diffusion and ELISA are suitable for use in routine serological tests.
Relationships
The virus differs from all the known small isometric plant viruses that sediment as a single component in having polypeptides that migrate as two components in SDS/polyacrylamide gel electrophoresis. In gel diffusion tests (Kenten & Jones, 1979), purified particles of strain T failed to precipitate with antisera to 21 other morphologically similar viruses: Andean potato latent, Andean potato mottle, arracacha A, carnation Italian ringspot, carnation mottle, carnation ringspot, cherry rasp leaf, cymbidium ringspot, narcissus tip necrosis, pelargonium flower break, pelargonium leaf curl, pelargonium ringspot, pelargonium ring pattern, red clover necrotic mosaic, saguaro cactus, southern bean mosaic, sowbane mosaic, tomato bushy stunt, turnip crinkle, turnip rosette and turnip yellow mosaic. The virus therefore seems to be the first representative of a new group. Strains T and O are serologically only distantly related, homologous and heterologous titres differing by 6-7 twofold dilution steps in gel diffusion tests and pronounced spurs forming (Fig. 5) where T and O precipitin lines intersect (Jones & Kenten, 1981; Jones, 1981).
Stability in Sap
In sap from infected C. quinoa leaves, infectivity is lost after dilution to 10-4 or occasionally to 10-5 or after heating for 10 min at 65-70°C, but is retained for up to 19 days at 20°C (Kenten & Jones, 1979; Jones & Kenten, 1981).
Purification
Extract infected C. quinoa, T. expansa or N. clevelandii leaves in a mixture of chloroform and 0.5 M phosphate buffer (pH 7.5) containing 0.2% mercaptoethanol and 0.05 M sodium ethylenediamine-tetraacetate (1 g leaf : 1 ml chloroform : 2 ml buffer), followed by two precipitations with polyethylene glycol and three cycles of differential centrifugation. Up to 150 A1cm,260 nm units of purified virus can be obtained per 1 kg of infected leaf by this procedure (Kenten & Jones, 1979).
Properties of Particles
In sucrose density gradients, purified virus preparations sediment as a single component with a sedimentation coefficient (s°20,w) of 126 S (Fig. 6).
A260/A280: 1.80 (strain T),
1.85 (strain O).
Buoyant density in CsCl (g/cm3): two components (Fig. 7),
1.481 and 1.492 (strain T), 1.484 and 1.495 (strain O).
Particle Structure
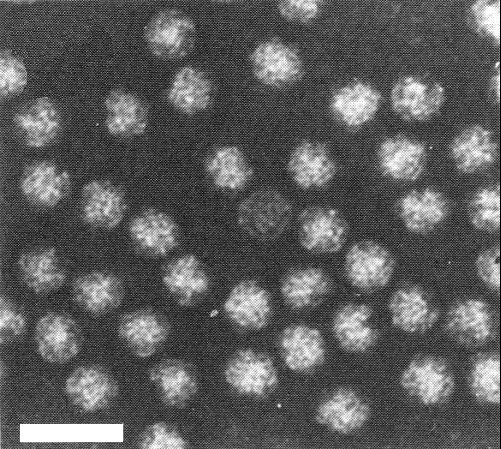
Particles are isometric, c. 26 nm in diameter with a hexagonal profile (Fig. 8). Detailed structure of the particles is not known.
Particle Composition
Nucleic acid: From the buoyant densities in CsCl, the nucleic acid contents of the two components were estimated to be 40 and 41% respectively.
Protein: Two coat protein components. A major one of M. Wt c. 26,000 and a minor one of M. Wt c. 20,000 (strain T) or c. 22,000 (strain O), estimated by electrophoresis in SDS/polyacrylamide gels. The ratio of the two was approximately 3:1 (Kenten & Jones, 1979; Jones & Kenten, 1981).
Relations with Cells and Tissues
No information.
Notes
Arracacha virus B and arracacha virus A have similar particle size, shape and properties in sap and both can occur together in the same arracacha plant. However, they can be readily distinguished because they are serologically unrelated and only arracacha virus A causes systemic necrosis in Chenopodium quinoa and Tetragonia expansa (Jones & Kenten 1978, 1981; Kenten & Jones, 1979). Arracacha virus B differs from all other isometric viruses found in potato in the Andes (e.g. Andean potato latent virus, Andean potato mottle virus, potato virus U, potato black ringspot and its close serological relative, the potato calico strain of tobacco ringspot virus). It is serologically unrelated to them and can be readily distinguished from them by differences in symptomatology in indicator hosts: only the three last-named viruses resemble arracacha virus B in causing systemic necrosis in C. murale and they all differ from it in causing systemic necrosis in C. quinoa (Jones, Fribourg & Slack, 1981). No other isometric virus has been described from oca.
Figures
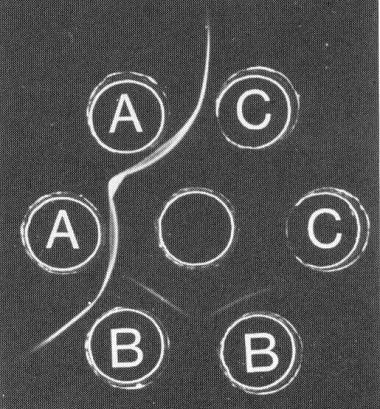
Gel-diffusion serological test. Centre well contained antiserum to strain O; wells A, B and C contained strain O, strain T and healthy sap respectively.
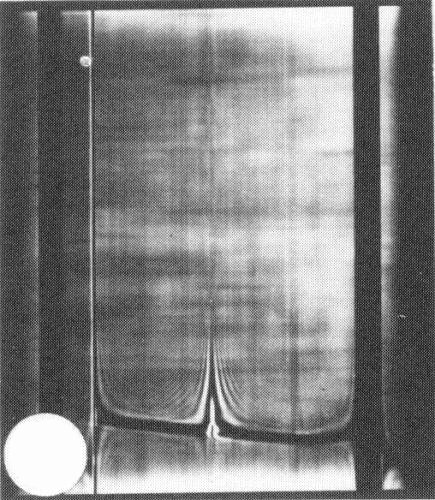
Schlieren pattern produced by a preparation of strain T after centrifugaton for 12 min at 29,500 rev/min. Schlieren angle 40° sedimentation from left to right.
References list for DPV: Arracacha virus B (270)
- Atkey & Brunt, Phytopath. Z. 103: 294, 1982.
- Jones, Pl. Dis. 65: 753, 1981.
- Jones, Ann. appl. Biol. 100: 315, 1982.
- Jones & Kenten, Ann. appl. Biol. 90: 85, 1978.
- Jones & Kenten, Phytopath. Z. 100: 88, 1981.
- Jones, Fribourg & Slack, Plant Virus Slide Series, No. 2, Am. Phytopath. Soc., 1981.
- Kenten & Jones, Ann. appl. Biol. 93: 31, 1979.