Details of DPV and References
DPV NO: 282 July 1984
Family: Potyviridae
Genus: Potyvirus
Species: Zucchini yellow mosaic virus | Acronym: ZYMV
Zucchini yellow mosaic virus
V. Lisa Istituto di Fitovirologia applicata del C.N.R., Torino, Italy
H. Lecoq Station de Pathologie Vegetale INRA, Montfavet, France
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by Lisa et al. (1981).
- Synonym
- Muskmelon yellow stunt virus (Rev. Pl. Path. 61: 3749)
- A virus with flexuous filamentous particles about 750 nm long consisting of single-stranded RNA and one coat protein, easily sap transmitted to a moderately large range of hosts and transmitted by aphids in the non-persistent manner. It causes economically important diseases in cucurbit crops in several Mediterranean countries, Central Europe and the USA.
Main Diseases
Severe diseases consisting of mosaic, yellowing, shoestringing, stunting, and fruit and seed deformations (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6) are produced in zucchini squash (Cucurbita pepo), muskmelon (Cucumis melo), cucumber (Cucumis sativus) and watermelon (Citrullus lanatus).
Geographical Distribution
Reported in France, Germany, Italy, Israel, Lebanon, Morocco, Spain, the USA (Lecoq, Lisa & Dellavalle, 1983; Lesemann et al., 1983), Egypt (Provvidenti, Gonsalves & Humaydan, 1984), Australia (R. S. Greber, personal communication), Mauritius (L. Bos and M. I. Dossa, personal communication) and the UK (D. M. Wright, personal communication). Isolated from the wild perennial cucurbit Melothria pendula in Florida (Adlerz et al., 1983b).
Host Range and Symptomatology
The experimental host range includes members of the families Aizoaceae, Amaranthaceae, Chenopodiaceae, Compositae, Cucurbitaceae, Labiatae, Leguminosae, Ranunculaceae, Scrophulariaceae, Solanaceae and Umbelliferae (Lecoq, Pitrat & Clement, 1981; Lisa et al., 1981).
- Diagnostic species
- Chenopodium amaranticolor, C. quinoa
. Chlorotic local lesions; no systemic infection. - Cucumis melo (muskmelon). Chlorotic local lesions; systemic vein clearing, yellowing,
mosaic, leaf deformation, stunting and occasional necrosis (Fig. 2, Fig. 3).
- Cucurbita okeechobeensis. Systemic mosaic. Not susceptible to most cucumber mosaic virus isolates.
- C. pepo (zucchini squash). Chlorotic local lesions; systemic vein netting, yellowing, mosaic and leaf deformation (Fig. 1), often followed by necrosis and premature death of the plant.
- Gomphrena globosa. Well-defined local lesions; no systemic infection. Not infected by squash mosaic virus.
- Lavatera trimestris. No infection. Watermelon mosaic virus 2 induces necrotic local lesions.
- Luffa acutangula. Systemic mosaic or latent infection.
- Ranunculus sardous. Systemic latent infection with most isolates. Not infected by either the watermelon mosaic virus 1 strain of papaya ringspot virus or watermelon mosaic virus 2.
- Cucurbita okeechobeensis. Systemic mosaic. Not susceptible to most cucumber mosaic virus isolates.
- Propagation species
- The virus is best maintained in Cucurbita pepo, which is also a good source of virus for purification.
- Assay species
- Chenopodium amaranticolor
is a useful local lesion assay host.
Strains
The virus is remarkably variable and several strains differing in symptomatology have been described. Lecoq et al. (1981) reported a strain differing from the type in inducing necrotic and wilting reactions in muskmelon cultivars, such as cv. Doublon, carrying the semi-dominant gene Fn (Risser et al., 1981). Later, 22 isolates of the virus were grouped into three pathotypes according to the reaction induced on muskmelon line PI 414723, which is resistant to some isolates of the virus (Pitrat & Lecoq, 1984; H. Lecoq & M. Pitrat, unpublished data). A strain causing symptoms in squash similar to those attributed to the watermelon mosaic virus 1 strain of papaya ringspot virus has been reported in the USA (Provvidenti et al., 1984) and one giving symptoms in muskmelon similar to those usually associated with watermelon mosaic virus 2 has been found in France (H. Lecoq, unpublished data).
Transmission by Vectors
Transmitted in the non-persistent manner by Aphis citricola (Purcifull et al., 1984), A. gossypii, Myzus persicae (Lecoq et al., 1981; Lisa et al., 1981) and Macrosiphum euphorbiae (H. Lecoq, unpublished data). About 20-30% of A. citricola and M. persicae transmitted the virus when tested individually following fasting for 2 h, acquisition access for 10 to 60 s and inoculation access for more than 1 h (Lisa et al., 1981; Purcifull et al., 1984). Transmission frequencies of about 80% were obtained when groups of three viruliferous A. gossypii or M. persicae were deposited per plant after a 3 min acquisition access time (Lecoq et al., 1981). Purified virus is not acquired through a Parafilm membrane by M. persicae unless a soluble fraction from infected plants, similar to the helper component described for other potyviruses (Govier & Kassanis, 1974; Pirone, 1977) is added (H. Lecoq, unpublished data).
Transmission through Seed
None detected in 1000 muskmelon seedlings grown from seed produced by infected plants (Lecoq et al., 1981).
Serology
The virus is a good immunogen and antisera with titres up to 1/4096 in the slide precipitin test have been obtained (Lecoq et al., 1983). The virus can easily be detected in crude plant sap by immunodiffusion in agar gel containing sodium dodecyl sulphate (SDS), by enzyme-linked immunosorbent assay (ELISA) and by immunoelectron microscopy (Lecoq et al., 1983; Lesemann et al., 1983; Makkouk & Abbasher, 1983; Purcifull et al., 1984).
Relationships
No significant serological differences have been detected between the isolate of the virus described in Italy and those reported elsewhere (Lecoq et al., 1983; Lesemann et al., 1983; Purcifull et al., 1984; Provvidenti et al., 1984). Cross-protection between strains was observed in muskmelon (Lecoq et al., 1981). The virus is serologically distantly related to watermelon mosaic virus 2, to bean yellow mosaic virus (Lecoq et al., 1981; Lisa et al., 1981; Adlerz et al., 1983a; Lesemann, 1983; Lesemann et al., 1983; Makkouk & Abbasher, 1983; Purcifull et al., 1984) and to amaranthus leaf mottle virus (Lovisolo & Lisa, 1976; V. Lisa & G. D’Agostino, unpublished data).
Stability in Sap
Infectivity was retained in infected zucchini squash sap after heating for 10 min at 55°C but not at 60°C; after dilution in distilled water up to 10-4 but not 10-5 and after 3 but not after 5 days storage at room temperature (V. Lisa & G. Dellavalle, unpublished data).
Purification
Homogenise infected zucchini squash plantlets in 0.5 M di-potassium hydrogen phosphate containing 0.02 M sodium sulphite, 0.01 M sodium diethyldithiocarbamate and 0.005 M di-sodium ethylenediamine-tetraacetate, pH 8.5, and emulsify the extract with Freon 113 (1,1,2-trifluoro-1,2,2-trichloroethane). Centrifuge at low speed and sediment the virus particles in the aqueous phase by high speed centrifugation. Resuspend the pellets in 0.05 M sodium citrate-0.02 M sodium sulphite, adjusted to pH 7.5 with citric acid, and further purify the virus by sucrose density gradient centrifugation (Lisa et al., 1981). Alternatively, the extract can be clarified with carbon tetrachloride; clarified or partially purified preparations can be treated with non-ionic detergents such as Triton X-100; centrifugation in a preformed linear gradient of 10-50% caesium sulphate or an equilibrium centrifugation in caesium sulphate can be used in place of the sucrose density gradient (H. Lecoq & V. Lisa, unpublished data). The virus has also been purified by a silver nitrate-polyethylene glycol method (Lesemann et al., 1983) and by concentration with polyethylene glycol and centrifugation to equilibrium in caesium chloride (Purcifull et al., 1984).
Properties of Particles
(Lisa et al., 1981). Upon isopycnic centrifugation in caesium chloride in 0.01 M phosphate buffer, pH 7, the virus forms a single homogeneous band with buoyant density 1.323 g/cm3 at 10°C.
A260/A280: 1.13.
Amax/Amin: 1.07.
Particle Structure
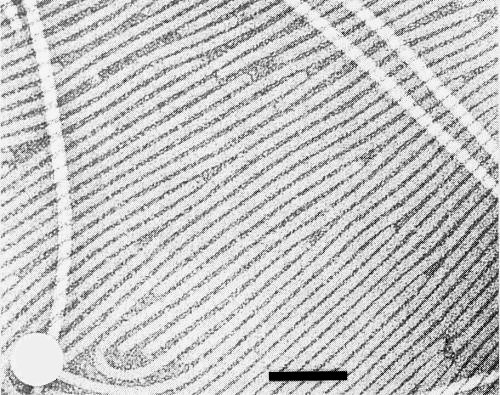
In crude zucchini squash sap, negatively stained with uranyl acetate, the particles are flexuous filaments with a modal length of about 750 nm (Lisa et al., 1981). Purified virus particles have typical potyvirus structure (Fig. 7, Fig. 8).
Particle Composition
Nucleic acid: single-stranded positive-sense RNA, of apparent M. Wt 2.93 x 106 as determined in 2.4% polyacrylamide gels in non-denaturing conditions (Lisa et al., 1981).
Protein: in SDS-polyacrylamide gels the virus coat protein migrated as a single band of estimated M. Wt 3.6 x 104 (Lisa et al., 1981).
Relations with Cells and Tissues
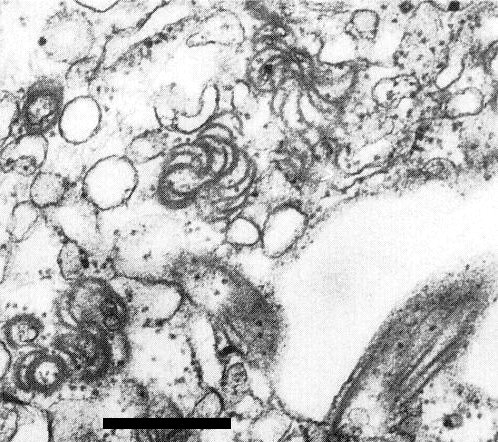
The virus induces cytoplasmic pinwheel inclusions and scrolls (Fig. 9), but not laminated aggregates (Lisa et al., 1981). Accumulation of endoplasmic reticulum and vesicles containing fibrillar material has also been noted (Lesemann et al., 1983).
Notes
Some zucchini yellow mosaic virus isolates induce symptoms similar to those attributed to other major cucurbit viruses, such as cucumber mosaic virus, the watermelon mosaic virus 1 strain of papaya ringspot virus, watermelon mosaic virus 2 and squash mosaic virus; a diagnosis based on symptoms is therefore hazardous. However, the virus can be easily identified in sap of field plants by standard serological techniques. Zucchini yellow mosaic virus frequently occurs together with these other common cucurbit viruses, from which it can usually be separated by using differential hosts, such as Cucurbita okeechobeensis and Ranunculus sardous. Separation from squash mosaic virus can be achieved by aphid transmission or by passage through Gomphrena globosa. Zucchini yellow fleck virus, a potyvirus also present in the Mediterranean basin (Vovlas, Hiebert & Russo, 1981), is not serologically related to zucchini yellow mosaic virus (M. Russo, personal communication).
Figures
References list for DPV: Zucchini yellow mosaic virus (282)
- Adlerz, Purcifull, Simone & Hiebert, Abstr. 4th int. Congr. Pl. Path., Melbourne, 1983: Abstr. no. 440, 1983a.
- Adlerz, Purcifull, Simone & Hiebert, Proc. Fla St. hort. Soc. 96: 72, 1983b.
- Govier & Kassanis, Virology 61: 420, 1974.
- Lecoq, Pitrat & Clement, Agronomie 1: 827, 1981.
- Lecoq, Lisa & Dellavalle, Pl. Dis. 67: 824, 1983.
- Lesemann, Acta Hort. 127: 159, 1983.
- Lesemann, Makkouk, Koenig & Natafji Samman, Phytopath. Z. 108: 304, 1983.
- Lisa, Boccardo, D'Agostino, Dellavalle & d'Aquilio, Phytopathology 71: 667, 1981.
- Lovisolo & Lisa, Poljopr. znanst. Smotra 39(49): 553, 1976.
- Makkouk & Abbasher, Abstr. 4th int Congr. Pl. Path., Melbourne, 1983: Abstr. no. 472, 1983.
- Pirone, in Aphids as Virus Vectors, p. 221, ed. K. Harris & K. Maramorosch, New York: Academic Press, 559 pp., 1977.
- Pitrat & Lecoq, Euphytica 33: 57, 1984.
- Provvidenti, Gonsalves & Humaydan, Pl. Dis. 68: 443, 1984.
- Purcifull, Adlerz, Simone, Hiebert & Christie, Pl. Dis. 68: 230, 1984.
- Risser, Pitrat, Lecoq & Rode, Agronomie 1: 835, 1981.
- Vovlas, Hiebert & Russo, Phytopath. Medit. 20: 123, 1981.