Details of DPV and References
DPV NO: 290 July 1984
Family: Secoviridae
Genus: Nepovirus
Species: Tomato ringspot virus | Acronym: ToRSV
This is a revised version of DPV 18
Tomato ringspot virus
R. Stace-Smith Agriculture Canada Research Station, 6660 N. W. Marine Drive, Vancouver, B.C., Canada V6T 1X2
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by Price (1936).
- Selected synonyms
- Tobacco ringspot virus No. 2 (Rev. appl. Mycol. 15: 831)
- Nicotiana virus 13 (Rev. appl. Mycol. 36: 303)
- Annulus zonatus (Rev. appl. Mycol. 28: 514)
- Peach yellow bud mosaic virus (Rev. appl. Mycol. 24: 65)
- Grape yellow vein virus (Rev. appl. Mycol. 35: 286)
- Nicotiana virus 13 (Rev. appl. Mycol. 36: 303)
- A virus with isometric particles about 28 nm in diameter sedimenting as three components and containing RNA as a bipartite genome. It is readily transmissible by inoculation of sap and has a wide host range, including both woody and herbaceous plants. It is transmitted by the nematode Xiphinema americanum and other closely-related Xiphinema spp. Reports of natural spread are largely confined to North America but the virus has been disseminated to many other countries in infected planting material.
Main Diseases
The virus occurs in nature mostly in perennial crops. It causes serious disease problems in those regions of North America where the nematode vectors also occur. The most serious diseases are yellow bud mosaic in peach and other Prunus spp. (Schlocker & Traylor, 1976), stem pitting and decline in peach, cherry and other Prunus spp. (Mircetich & Civerolo, 1972; Smith, Stouffer & Soulen, 1973; Mircetich & Fogle, 1976), ‘brownline’ disease of prune (Hoy & Mircetich, 1984), union necrosis and decline in apple (Stouffer, Hickey & Welsh, 1977), ringspot and decline in red raspberry (Stace-Smith, 1984) and decline in grapevine (Allen & van Schagen, 1982). Most infected plants show distinctive symptoms as a shock reaction; chronically infected plants usually exhibit no obvious symptoms but show a general decline in productivity.
Geographical Distribution
The virus appears to be endemic in North America, where it occurs in pockets in the region around the Great Lakes and in the Pacific seaboard from California to British Columbia. Natural spread is probably confined to those areas where there are moderate to high populations of nematode vectors belonging to the genus Xiphinema. The virus has been isolated from ornamentals and berry crops in other parts of the world and there is limited evidence of field spread associated with occurrences in Japan (Iwaki & Komuro, 1974) and the USSR (Gordejchuk et al., 1977). Isolations have been made from narcissus in Japan (Iwaki & Komuro, 1971); pelargonium in Sweden (Rydén, 1972), United Kingdom (Hollings, Stone & Dale, 1972), Netherlands (Maat & van Hoof, 1974) and Denmark (Christensen & Paludan, 1978); raspberry in Yugoslavia (Jordovic, Rankovic & Dimitrijevic 1973), the USSR (Gordejchuk et al., 1977) and Chile (Auger & Converse, 1982); and redcurrant in New Zealand (Fry & Wood, 1978) and the USSR (Gordejchuk et al., 1977).
Host Range and Symptomatology
Experimental host range is wide; species in more than 35 dicotyledonous and monocotyledonous families are susceptible. In nature, the virus occurs mostly in ornamentals and woody or semi-woody plants. Transmissible by inoculation with sap, readily to herbaceous hosts but with difficulty to woody hosts.
- Diagnostic species
- Chenopodium amaranticolor and C. quinoa. Small chlorotic or
necrotic local lesions (Fig. 1); systemic apical necrosis.
- Cucumis sativus (cucumber). Local chlorotic spots; systemic chlorosis and mottle.
- Lycopersicon esculentum (tomato). Local necrotic flecks; systemic mottle and necrosis.
- Nicotiana tabacum (tobacco). Necrotic local spots or rings (Fig. 2); systemic necrotic or chlorotic ring and line patterns. Leaves produced later are symptomless but contain virus.
- Petunia hybrida (Fig. 3). Local necrotic lesions; necrotic collapse of young systemically infected leaves.
- Phaseolus vulgaris (bean). Chlorotic local lesions; systemic rugosity and necrosis of tip leaves.
- Vigna unguiculata (cowpea). Chlorotic or necrotic local lesions (Fig. 4); most isolates cause systemic tip necrosis.
- Cucumis sativus (cucumber). Local chlorotic spots; systemic chlorosis and mottle.
- Propagation species
- Nicotiana spp. or woody plants such as raspberry or redcurrant are
suitable for maintaining cultures; Cucumis sativus (cucumber),
Nicotiana clevelandii or Petunia hybrida are good sources of virus
for purification.
- Assay species
- Chenopodium amaranticolor, C. quinoa, Nicotiana tabacum and Vigna unguiculata are useful local lesion hosts. Cucumber is useful as a source and bait plant for nematode transmission experiments (Téliz, Grogan & Lownsbery, 1966).
Strains
Variants giving slightly different symptoms in herbaceous hosts have been found. The best known variants, distinguished not by reactions in experimental hosts but by the diseases they cause in the field, are as follows:
Tobacco strain = tobacco ringspot virus No. 2 of Price (1936). The type strain. Occurred ‘spontaneously’ in tobacco seedlings in a greenhouse. Source eastern USA.
Peach yellow bud mosaic strain. Occurs naturally in almond, apricot and peach. Source western USA. This strain may not really be distinct from Price's strain because the two viruses cause similar symptoms in herbaceous hosts (Karle, 1960) and cannot be distinguished serologically (Cadman & Lister, 1961). Moreover, most if not all isolates of the virus that have been tested can cause yellow bud mosaic symptoms in peach.
Grape yellow vein strain. Occurs naturally in grape. Causes reactions in herbaceous hosts similar to those caused by the above strains but differs in causing top necrosis in cowpea; also differs somewhat serologically (Gooding, 1963). Source western USA.
Other variants. Some serological variability is reported among isolates from Prunus (prune brown line, prunus stem pitting and cherry leaf mottle isolates), but the extent of these differences is not clear; however, the cherry leaf mottle isolate was less efficiently transmitted than the other two isolates by Xiphinema californicum (Hoy, Mircetich & Lownsbery, 1984).
Transmission by Vectors
Most vector studies have been done with nematode populations identified as Xiphinema americanum. However, X. americanum sensu lato is now considered to be a complex of many species (Lamberti & Bleve-Zacheo, 1979) and clarification of the vector potential of the component species is required. In addition to X. americanum, X. rivesi is regarded as an important vector of the virus in eastern USA (Forer & Stouffer, 1982; Rosenberger, Harrison & Gonsalves, 1983; Mountain et al., 1983), and X. californicum in western USA (Hoy et al., 1984). X. brevicolle has been recorded as a vector in Germany (Fritzsche & Kegler, 1968) but the evidence supporting this claim is considered to be inadequate (Trudgill, Brown & McNamara, 1983). The two-spotted mite failed to act as a vector (Granillo & Smith, 1974). The virus is transmitted by both the adult and larval stages of X. americanum (sensu lato), is acquired within 1 h and can be inoculated into healthy plants within 1 h (Téliz et al., 1966; Iwaki & Komuro, 1974). In raspberry plantings, the average rate of spread is c. 2 m per year (Converse & Stace-Smith, 1971).
Transmission through Seed
Varying degrees of seed transmission have been reported in a range of test species, including soybean (Kahn, 1956), strawberry (Mellor & Stace-Smith, 1963), raspberry (Braun & Keplinger, 1973), pelargonium (Scarborough & Smith, 1977) and dandelion (Mountain et al., 1983). Pollen transmission to seed has been demonstrated in pelargonium (Scarborough & Smith, 1977).
Transmission by Dodder
A virus thought to be tomato ringspot virus was not transmitted by Cuscuta californica, C. subinclusa or C. campestris (Bennett, 1944). A virus from Euonymus spp., initially designated euonymus ringspot virus (Puffinberger & Corbett, 1973) but later found to be an isolate of tomato ringspot virus (C. W. Puffinberger, personal communication), was transmitted by C. campestris.
Serology
The virus is a good immunogen. Rabbits given a series of intravenous and intramuscular injections of 0.1 mg virus at weekly intervals for 4 wk yielded antiserum samples with titres of 1/512 - 1/1024 in agar gel double diffusion tests (R. Stace-Smith, unpublished data). A single precipitin band is formed. Visible precipitin bands may be obtained with undiluted crude sap from cucumber cotyledons with symptoms but the test is not reliable. The virus is readily detected in woody hosts by ELISA (Converse, 1978; Allen & van Schagen, 1982; Rosenberger et al., 1983; Barrat, Scorza & Otto, 1984) but some strains may be missed because of the high serological specificity of double antibody sandwich ELISA (Stace-Smith, 1984). Serologically distinguishable strains have been identified in agar gel double diffusion tests (Gooding, 1963; Hoy et al., 1984). The degree of relatedness can be assessed by using an enzyme-linked immunosorbent blocking assay (Powell & Derr, 1983).
Relationships
The virus is a distinctive member of the nepovirus group and is unrelated serologically to any other member. However, it is not readily distinguished from several of the nepoviruses on the basis of host range and symptomatology. The virus differs from many members of the nepovirus group by the high M. Wt of its RNA-2, which results in the middle and bottom nucleoprotein components having similar sedimentation coefficients. On this basis, tomato ringspot virus could be assigned to a sub-group that also contains grapevine Bulgarian latent, blueberry leaf mottle, peach rosette mosaic, cherry leaf roll, lucerne Australian latent and chicory yellow mottle viruses (Harrison & Murant, 1977; Martelli et al., 1978; Murant, 1981). One way cross-protection against cherry leaf roll virus was demonstrated by Varney & Moore (1952) and Fulton & Fulton (1970). However, tomato ringspot virus is not serologically related to cherry leaf roll virus (Fulton & Fulton, 1970) or to other nepoviruses with similar properties (Jones & Duncan, 1980).
Stability in Sap
The virus is infective in sap of tobacco and cucumber at dilutions of 10-2 and occasionally at 10-3. The virus in crude sap loses infectivity after 10 min at about 58°C, 2 days at 20°C, 3 weeks at 4°C, or several months at -20°C (Stace-Smith, 1962). Infectivity may be retained in lyophilised sap for up to 4 yr (Hollings & Stone, 1970).
Purification
The virus has been purified by several different procedures and from several different host species. Yields vary somewhat depending upon the virus isolate, the host and the procedure selected for purification but an average yield is c. 1.0 mg/100 g leaf tissue. Infected cucumber tissue is the preferred virus source (Stace-Smith, 1966; Uyemoto, 1970; Schneider, White & Civerolo, 1974; Allen & Dias, 1977) but tobacco (Goff & Corbett, 1977) and petunia (Powell & Derr, 1983) have also been used. The extraction buffer is not critical: 0.5 M sodium borate-boric acid buffer, pH 6.7 (Stace-Smith, 1966), 0.05 M potassium citrate, pH 6.5 (Powell & Derr, 1983), or 0.05 M potassium phosphate, pH 7.0 (Schneider et al., 1974), each containing antioxidants, have been used. Partial clarification is achieved by freezing buffered extracts or by freezing infected tissue before extraction (Gooding, 1963; Powell & Derr, 1983; Stace-Smith, 1966; Schneider et al., 1974). Further clarify the extract by adding 15 g of granular ammonium sulphate per 100 ml (Stace-Smith, 1966), then concentrate the virus by two cycles of differential centrifugation. Irrespective of the purification procedure that is used, the pellet contains contaminating host materials and, as a final purification step, the concentrated virus should be subjected to sucrose density gradient centrifugation (Fig. 5).
Properties of Particles
In sucrose density gradients, the virus particles sediment as three components, designated top (T), middle (M) and bottom (B) (Schneider et al., 1974; Allen & Dias, 1977).
Sedimentation coefficients, s20,w: 53 S (T), 119 S (M),
127 S (B). The T component consists of protein subunits and lacks RNA (Fig. 6).
The ratio of M to B particles is approximately 1:1 in six isolates that have been
purified (R. Stace-Smith, unpublished data). The M and B components form two
closely-spaced bands following density gradient centrifugation or sedimentation
in caesium chloride. The particles comprising these bands have not been separated
completely but infectivity is enhanced by mixing partially-separated M and B
components, indicating that both particle types are probably necessary for
infection (Schneider et al., 1974; Allen & Dias, 1977; Jones &
Duncan, 1980).
A260/A280=0.9 (T), 1.8 (combined M and B).
Absorbance at 260 nm (1 mg/ml, 1 cm light path): 10.0 (M and B components combined).
Buoyant densities in CsCl at 25°C (g/cm3): 1.495 (M) and
1.51(B) (Schneider et al., 1974).
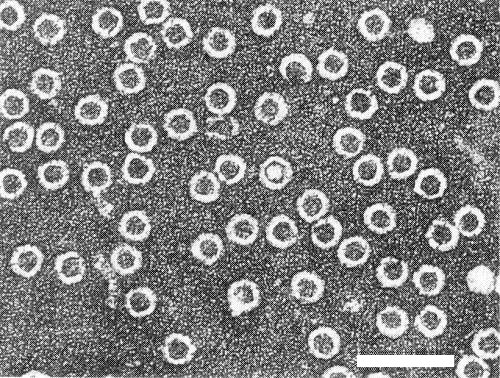
Particle Structure
Particles are isometric, c. 28 nm in diameter, with angular outlines (Fig. 7). T particles are penetrated by negative stain (Fig. 6).
Particle Composition
Nucleic acid: RNA, single-stranded. Two species of M. Wt c. 2.8 x 106 and 2.4 x 106; s20,w of 32.6 and 30.9, respectively. Molar percentages of nucleotides: G 26; A 23; C 22; U 29. The nucleic acid contents of the M and B components are c. 41 and 44% respectively (Tremaine & Stace-Smith, 1968; Schneider et al., 1974; Allen & Dias, 1977; Murant & Taylor, 1978; Murant et al., 1981).
Proteins: In 10% polyacrylamide gels containing 0.1% SDS the coat protein migrates as one component of M. Wt c. 58,000 (Allen & Dias, 1977). Like other nepoviruses, tomato ringspot virus probably possesses a genome-linked protein because infectivity of RNA is destroyed by treatment with proteinase K (Mayo, Barker & Harrison, 1982).
Relations with Cells and Tissues
The virus occurs in all parts of infected plants but detection in ultrathin sections by electron microscopy is difficult. Particles were observed in plasmodesmata and cytoplasm but not in the nuclei of infected Datura stramonium (De Zoeten & Gaard, 1969). In infected Nicotiana clevelandii, particles are found in plasmodesmata and, when these are observed, there is a vacuole in the cytoplasm adjacent to the plasmodesma (R. Stace-Smith & B. Valentine, unpublished data). No changes were detected by light microscopy in vegetative tissues of infected pelargonium but pollen grain abortion, abnormal ovules, aborted ovules and tissue disintegration were detected in infected florets (Murdock, Nelson & Smith, 1976). In red raspberry, reduced drupelet set and pollen abortion is common in some infected cultivars (Daubeny, Freeman & Stace-Smith, 1975).
Notes
The geographical distribution, natural host range and vector relations of tomato ringspot virus closely parallel those of tobacco ringspot virus. Differential host reactions are useful for distinguishing isolates of the two viruses but such tests cannot reliably distinguish all isolates. Serological tests are essential for positive identification of isolates that are thought to belong to viruses in the nepovirus group. Serology is particularly useful in identifying strains of tomato ringspot virus because all known strains cross-react in agar gel double diffusion tests and there appears to be no cross-reactivity between it and other nepoviruses. The virus should be partially purified and concentrated for reliable detection by agar gel double diffusion tests.
In the early literature, the name ‘tomato ringspot’ was applied to two
unrelated viruses, one isolated from tobacco seedlings by Price (1936) and
the other from tomato plants by
Samson & Imle (1942). Price’s isolate,
or isolates serologically related to it, have retained the designation ‘tomato
ringspot’; the ‘tomato ringspot’ virus of Samson & Imle (1942) was probably
the same as the ‘tomato top necrosis’ virus of Bancroft (1968) but both isolates
are now lost. Elm mosaic virus was at one time thought to be a strain of tomato
ringspot virus (Varney & Moore, 1952) but is now known to be a strain of
cherry leaf roll virus (Jones & Murant, 1971).
Tomato ringspot virus is associated with diseases of commercial fruit and
ornamental crops in northeastern USA, southeastern Canada and western USA.
Prunus stem pitting and apple union necrosis and decline are of prime importance
in eastern North America whereas peach yellow bud mosaic and red raspberry
ringspot are serious disease problems in the west. The fact that the virus is
widespread in perennial plant species and causes a severe decline in productivity
makes it one of the more damaging plant viruses in North America. The virus is
prevalent in dandelion and may be disseminated over considerable distances in
windblown seeds (Rosenberger et al., 1983). Furthermore, dandelions and
other perennial weeds provide reservoirs for the virus. Transmission to crop
hosts appears to be exclusively by nematode vectors.
Control of tomato ringspot virus in established plantings of fruit tree or
berry crops is difficult. The disease is usually not obvious at an early stage
of development so that extensive indexing is required to determine the incidence
and distribution of infection. Plants indexing positive may be removed and
replanted but, unless measures are taken to destroy nematode vectors, the virus
would soon be transmitted to healthy plants in replanted areas. A more effective
course might be to delay action until the yield loss resulting from infection is
sufficient to warrant complete removal of the crop and then to treat the soil
with a fumigant nematicide before replanting with certified virus-free stock.
However, experience with Xiphinema americanum transmitting peach rosette
mosaic virus in grapevine (Ramsdell et al., 1983) suggests that control
of this nematode adequate to prevent virus spread may be achieved only when the
soil is fumigated to a considerable depth (c. 90 cm). Very thorough
nematode control is presumably necessary because of the continuing presence of
virus sources in the form of infected weed seeds (Mountain et al., 1983).
Replanting with resistant cultivars, e.g. of grapevine (Allen, Dias & van
Schagen, 1982) or plum (Hoy & Mircetich, 1984) would probably offer a better
long-term approach.
Figures

Linear-log sucrose density gradient profile of a purified preparation of a raspberry isolate showing relative concentration of top (T), middle (M) and bottom (B) components. Gradient was centrifuged in a Beckman SW 41 rotor at 4°C, 38 000 rev/mm for 90 min.
References list for DPV: Tomato ringspot virus (290)
- Allen & Dias, Can. J. Bot. 55: 1029, 1977.
- Allen & van Schagen, Can. J. Pl. Path. 4: 272, 1982.
- Allen, Dias & van Schagen, Can. J. Pl. Path. 4: 275, 1982.
- Auger & Converse, Acta Hort. 129: 9, 1982.
- Bancroft, Phytopathology 58: 1360, 1968.
- Barrat, Scorza & Otto, Pl. Dis. 68: 198, 1984.
- Bennett, Phytopathology 44: 905, 1944.
- Braun & Keplinger, Pl. Dis. Reptr 57: 431, 1973.
- Cadman & Lister, Phytopathology 51: 29, 1961.
- Christensen & Paludan, J. hort. Sci. 53: 209, 1978.
- Converse, Pl. Dis. Reptr 62: 189, 1978.
- Converse & Stace-Smith, Phytopathology 61: 1104, 1971.
- Daubeny, Freeman & Stace-Smith, Can. J. Pl. Sci. 55: 755, 1975.
- De Zoeten & Gaard, J. Cell Biol. 40: 814, 1969.
- Forer & Stouffer, Pl. Dis. 66: 735, 1982.
- Fritzsche & Kegler, Biol. Zbl. 87: 139, 1968.
- Fulton & Fulton, Phytopathology 60: 114, 1970.
- Fry & Wood, N.Z. Jl agric. Res. 21: 543, 1978.
- Goff & Corbett, Phytopathology 67: 1096, 1977.
- Gooding, Phytopathology 53: 475, 1963.
- Gordejchuk, Krylov, Krylova & Samonina, Zentbl. Bakt. ParasitKde 132: 686, 1977.
- Granillo & Smith, Phytopathology 64: 494, 1974.
- Harrison & Murant, CMI/AAB Descr. Pl. Viruses 185, 4 pp., 1977.
- Hollings & Stone, Ann. appl. Biol. 65: 411, 1970.
- Hollings, Stone & Dale, Pl. Path. 21: 46, 1972.
- Hoy & Mircetich, Phytopathology 74: 272, 1984.
- Hoy, Mircetich & Lownsbery, Phytopathology 74: 332, 1984.
- Iwaki & Komuro, Ann. phytopath. Soc. Japan 37: 108, 1971.
- Iwaki & Komuro, Jap. J. Nematol. 4: 27, 1974.
- Jones & Duncan, J. gen. Virol. 50: 269, 1980.
- Jones & Murant, Ann. appl. Biol. 69: 11, 1971.
- Jordovic, Rankovic & Dimitrijevic, Jugost. Vocarstvo. 7:163, 1973.
- Kahn, Phytopathology 46: 295, 1956.
- Karle, Phytopathology 50: 466, 1960.
- Lamberti & Bleve-Zacheo, Nematol. Medit. 7: 51, 1979.
- Maat & van Hoof, Ann. Rep. Inst. phytopath. Res. Wageningen, 1973, pp. 31, 36, 1974.
- Martelli, Quacquarelli, Gallitelli, Savino & Piazzolla, Phytopath. Medit. 17: 145, 1978.
- Mayo, Barker & Harrison, J. gen. Virol. 59: 149, 1982.
- Mellor & Stace-Smith, Can. J. Bot. 41: 865, 1963.
- Mircetich & Civerolo, Phytopathology 62: 1294, 1972.
- Mircetich & Fogle, in Virus Diseases and Noninfectious Disorders of Stone Fruits in North America (Agriculture Handbook No. 437), p. 77, Washington, US Dept. Agric., 433 pp., 1976.
- Mountain, Powell, Forer & Stouffer, Pl. Dis. 67: 867, 1983.
- Murant, in Handbook of Plant Virus Infections and Comparative Diagnosis, p. 197, ed. E. Kurstak, Amsterdam: Elsevier/North-Holland, 943 pp., 1981.
- Murant & Taylor, J. gen. Virol. 41: 53, 1978.
- Murant, Taylor, Duncan & Raschké, J. gen. Virol. 53: 321, 1981.
- Murdock, Nelson & Smith, Phytopathology 66: 844, 1976.
- Powell & Derr, Phytopathology 73: 660, 1983.
- Price, Phytopathology 26: 665, 1936.
- Puffinberger & Corbett, Phytopathology 63: 804, 1973.
- Ramsdell, Bird, Gillett & Rose, Pl. Dis. 67: 625, 1983.
- Rosenberger, Harrison & Gonsalves, Pl. Dis. 67: 356, 1983.
- Rydén, Phytopath. Z. 73: 178, 1972.
- Samson & lmle, Phytopathology 32: 1037, 1942.
- Scarborough & Smith, Phytopathology 67: 292, 1977.
- Schlocker & Traylor, in Virus Diseases and Noninfectious Disorders of Stone Fruits in North America (Agriculture Handbook No. 437), p. 156, Washington: US Dept. Agric., 433 pp., 1976.
- Schneider, White & Civerolo, Virology 57: 139, 1974.
- Smith, Stouffer & Soulen, Phytopathology 63: 1404, 1973.
- Stace-Smith, Can. J. Bot. 40: 905, 1962.
- Stace-Smith, Virology 29: 240, 1966.
- Stace-Smith, Pl. Dis. 68: 274, 1984.
- Stouffer, Hickey & Welsh, Pl. Dis. Reptr 61: 20, 1977.
- Téliz, Grogan & Lownsbery, Phytopathology 56: 658, 1966.
- Tremaine & Stace-Smith, Virology 35: 102, 1968.
- Trudgill, Brown & McNamara, Revue Nematol. 6: 133, 1983.
- Uyemoto, Phytopathology 60: 1838, 1970.
- Varney & Moore, Phytopathology 42: 476, 1952.