Details of DPV and References
DPV NO: 291 July 1984
Family: Luteoviridae
Genus: Polerovirus
Species: Potato leafroll virus | Acronym: PLRV
This is a revised version of DPV 36
Potato leafroll virus
B. D. Harrison Scottish Crop Research Institute, Invergowrie, Dundee DD2 5DA, Scotland, UK
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Infectious nature of potato leafroll disease described by Quanjer,
Van der Lek & Oortwijn Botjes (1916), virus particles purified
by Peters (1967).
Selected synonyms
- Potato phloem necrosis virus (Quanjer, 1913)
- A virus with RNA-containing isometric particles c. 24 nm in diameter. Host range limited. Apparently confined to phloem tissue and not transmissible by inoculation with sap. Transmitted by several aphid species in the persistent manner. Occurs in most places where potatoes are grown.
Main Diseases
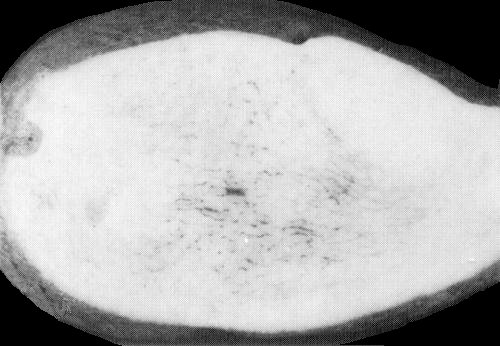
Causes an economically very important disease in potato (Solanum tuberosum ssp. tuberosum) in many countries. Symptoms of primary infection consist typically of pallor, and in some cultivars reddening, of the tip leaves, which may become rolled and assume an erect habit. Secondary symptoms, in plants grown from infected tubers, are stunting of the shoots and upward rolling of leaflets, especially those on lower leaves (Fig. 1), which break easily when crushed and may develop marginal necrosis. Upper leaves are slightly chlorotic. Carbohydrates accumulate in affected leaves because phloem transport is impaired. The virus is transmitted through a variable proportion of tubers on plants with primary infection and through all tubers on plants with secondary infection. In some cultivars (e.g. Golden Wonder and Russet Burbank), tubers on plants with either primary or secondary infection may develop internal net necrosis (Fig. 5).
In some cultivars of S. tuberosum ssp. andigena
grown in S. America, leaf rolling is not a typical symptom of
secondary infection, but the plants are stunted and their tip
leaves develop marginal yellowing (enanismo amarillo;
Fig. 2; Rodriguez & Jones, 1978).
In tomato, tomato yellow top strains cause stunting of plants,
marginal yellowing and curling of leaflets (Fig. 3), and death of
flower buds (Braithwaite & Blake, 1961).
Geographical Distribution
Occurs in potato in most places where the crop is grown. Tomato yellow top diseases are reported from N. America (Altstatt & Ivanoff, 1945), S. America (Costa, 1949) and Australasia (Sutton, 1955), but not all are known to be caused by strains of potato leafroll virus.
Host Range and Symptomatology
The virus is not transmitted by manual inoculation with sap but is transmissible by vector aphids and by grafting. Most known hosts (about 20 species) are in the Solanaceae. Non-solanaceous hosts include Amaranthus caudatus, Celosia argentea and Gomphrena globosa (Amaranthaceae), Nolana lanceolata (Nolanaceae) (Natti, Kirkpatrick & Ross, 1953), Capsella bursa-pastoris (Cruciferae; Thomas, 1984) and Montia perfoliata (Portulacaceae; Tamada, Harrison & Roberts, 1984).
- Diagnostic species
- Datura stramonium. Systemically infected leaves develop
interveinal yellowing.
- Physalis floridana. Plants become more or less stunted, and systemically infected leaves develop mild interveinal chlorosis (Fig. 4). Older leaves may become slightly rolled.
- Solanum tuberosum ssp. tuberosum (potato). Plants inoculated by aphids or by grafting develop the symptoms described under Main Diseases.
- Brassica pekinensis (Chinese cabbage), Raphanus sativus (radish) and Vicia faba (broad bean) are thought to be non-hosts.
- Physalis floridana. Plants become more or less stunted, and systemically infected leaves develop mild interveinal chlorosis (Fig. 4). Older leaves may become slightly rolled.
- Propagation species
- Virus cultures are readily maintained in potato clones; dormant
infected tubers can be stored at 4°C for more than a year.
Systemically infected leaves of P. floridana or potato are
suitable sources of virus for purification.
- Assay species
- P. floridana can be used as a test plant in aphid transmission experiments in which the aphids acquire the virus by feeding on plants, or by feeding through membranes on virus preparations, or by injection. Infectivity can also be measured by inoculating purified virus to mesophyll protoplasts obtained from tobacco (Takanami & Kubo, 1979a) or potato (Barker & Harrison, 1982), incubating the protoplasts for about 2 days and then determining the percentage that can be stained by fluorescent virus-specific antibody.
Strains
Strains from potato have been distinguished by the severity of symptoms induced in potato, P. floridana (Fig. 4; Webb, Larson & Walker, 1951) or Montia perfoliata, or by their ease of transmission by Myzus persicae (Tamada et al., 1984). These strains did not, however, differ antigenically (Tamada et al., 1984), and avirulent strains protected P. floridana plants from virulent strains (Webb, Larson & Walker, 1952; Harrison, 1958a).
Tomato yellow top strains (Thomas, 1984) differ from potato
strains in causing yellow edge symptoms in tomato leaves (Fig. 3)
and little or no symptom in potato, and in being readily
transmitted by the aphid Macrosiphum euphorbiae
(Braithwaite & Blake, 1961). Potato leafroll and tomato yellow
top isolates from Australia were antigenically indistinguishable
(Thomas, 1984) and they cross-protected in P. floridana
plants (J. E. Thomas, unpublished results).
Transmission by Vectors
Several aphid species are reported to transmit the virus (Kennedy, Day & Eastop, 1962). Myzus persicae is the most efficient and important vector; Macrosiphum euphorbiae transmits potato strains less efficiently but is a good vector of Australian tomato yellow top isolates. The minimum access times needed by M. persicae to acquire and to inoculate the virus are each about 1 h. There is a latent period and the minimum total time for transmission is about 12 h (Sugawara, Kojima & Murayama, 1974); however, transmission frequency increases with increase in the access periods up to 2 days or more. Larval and adult aphids can transmit the virus. Aphids remain infective after moulting and can retain infective virus for life. The virus can be detected in aphid haemolymph, and virus-free M. persicae become infective after virus containing preparations are injected into their haemocoeles (Heinze, 1955; Day, 1955).
The virus content of aphids decreases when they are kept on
immune plants (Harrison, 1958b). However, Stegwee &
Ponsen (1958) reported that the virus could be transferred many
times by serial injection of aphids kept on virus-immune plants,
although this was not confirmed by Sugawara, Kojima & Murayama
(1973) or Eskandari, Sylvester & Richardson (1979). Serological
assay of aphid extracts indicates that the virus content of aphids
increases with increase in acquisition access period up to about 7 days,
but decreases in a temperature dependent manner after the aphids are
removed from the virus source, at first more rapidly and later very
slowly (Tamada & Harrison, 1981). Despite this evidence, the
possibility of limited virus replication in aphids cannot be excluded.
Indeed, Weidemann (1982) found that virus particle antigen accumulated
in the nuclei of cells of the midgut and principal salivary gland of
M. persicae 1-2 days after the beginning of virus acquisition
and suggested that the virus multiplied in these cells. Gildow (1982)
detected virus-like particles in cells of the accessory salivary
glands of M. persicae, on their probable path from the
haemolymph to saliva.
Transmission through Seed
Not reported.
Serology
The virus particles are very immunogenic. Antisera with titres of 1/1000 in gel diffusion precipitin tests can be prepared; a single reaction line is obtained. Mouse monoclonal antibodies have been prepared to a Canadian isolate of the virus (Martin & Stace-Smith, 1984). Intracellular virus antigen can be detected with fluorescein-conjugated antibody (Kubo & Takanami, 1979). Because of the low concentration of virus antigen in tissues, sensitive serological techniques have proved especially valuable. ELISA can detect the virus reliably in potato leaves (Casper, 1977; Maat & de Bokx, 1978; Mehrad, Lapierre & Maury, 1978; Gugerli, 1979; Tamada & Harrison, 1980a), potato tubers and virus-carrying aphids (Clarke, Converse & Kojima, 1980; Gugerli, 1980; Tamada & Harrison, 1980b, 1981). Immunosorbent electron microscopy has been similarly successful (Kojima, Chou & Shikata, 1978; Roberts & Harrison, 1979; Van Balen, 1982).
Relationships
In its particle properties, tissue tropism and symptoms, and its transmission in the persistent manner by aphids, potato leafroll virus is a typical luteovirus. Relationships to several members of this group have been detected by gel-diffusion serological tests (Kubo & Takanami, 1978; T. Tamada, unpublished results), immunoelectron microscopy (Roberts, Tamada & Harrison, 1980) and/or by density gradient zone-depletion serological tests (Waterhouse & Murant, 1981). The closest relationships are to tobacco necrotic dwarf (SDI = 1), beet western yellows/beet mild yellowing (SDI = 2-4) and bean leaf roll viruses: however, antisera to potato leafroll virus react more strongly with particles of the beet viruses than do antisera to the beet viruses with particles of potato leafroll virus (Kubo & Takanami, 1978; Roberts et al., 1980; Kubo, 1981; Tamada et al., 1984; T. Tamada, unpublished results). Strains of beet western yellows virus differ in the extent of their serological relationship to tomato yellow top isolates of potato leafroll virus occurring in Australia (Thomas, 1984).
There is no evidence of antigenic variation among potato
isolates of potato leafroll virus (Kojima, 1981; Tamada
et al., 1984), but Thomas (1984) obtained results
suggesting that luteovirus isolates from tomato plants with yellow
top diseases in different countries may differ antigenically.
Stability in Sap
In Physalis floridana sap, the thermal inactivation point (10 min) was between 70° and 80°C, dilution end-point about 10-4, and longevity at 2°C between 5 and 10 days, when infectivity was assayed by the aphid injection method (Murayama & Kojima, 1965). Infectivity was retained for at least a year in leaf tissue stored at -70°C (T. Tamada & B. D. Harrison, unpublished results).
Purification
(Based on Takanami & Kubo, 1979a). Freeze infected Physalis floridana or potato leaves for a week or more at -70°C (-20°C may be adequate). Grind leaves (500 g) at room temperature with 1 litre 0.1 M sodium citrate buffer, pH 6.0, containing 0.5% 2-mercaptoethanol and 1% Driselase, then incubate at 25°C for 2-3 h with shaking. Adjust extract to pH 7.0 by adding 0.2 M Na2HPO4, emulsify with 0.67 vol. chloroform/butanol mixture (1:1, v/v) and centrifuge at 5000 rev./min for 15 min. To the aqueous phase, add polyethylene glycol, M. Wt 6000, to 8% (w/v) and NaCl to 0.2 M. Stir for 1 h at 4°C, incubate at room temperature for 1-2 h, then centrifuge at 15,000 rev./min for 15 min. Resuspend precipitates in 100 ml 0.02 M sodium phosphate buffer, pH 7.5, containing 1% Triton X-100, and centrifuge at 10,000 rev./min for 15 min. Further purify by two cycles of high-speed (using cushions of 20% sucrose) and low-speed centrifugation, resuspending the final high-speed sediments in 2 ml phosphate buffer. Further purification may be achieved by centrifugation in 10-40% sucrose gradients. Purified virus particles can be stored frozen but precipitate reversibly at about 4°C. All centrifugation is therefore done at 15°C. Virus yields are about 0.7 mg/kg potato leaf and 1 mg/kg P. floridana leaf.
Properties of Particles
Purified preparations contain one sedimenting component.
Sedimentation coefficient (s20,w): 115 S
(Takanami & Kubo, 1979a).
A260/A280: 1.78;
A260/A240: 1.43 (Takanami
& Kubo, 1979a).
A(0.1%;1 cm) at 260 nm: 8.6 (Takanami & Kubo, 1979a).
Buoyant density in CsCl: 1.39 g/cm3 (Rowhani &
Stace-Smith, 1979).
Buoyant density in Cs2SO4: 1.34 g/cm3 (Thomas, 1984).
Particle Structure
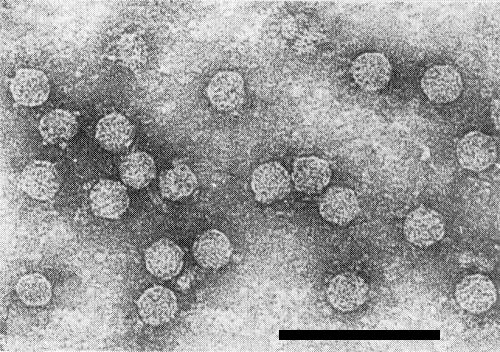
Particles are isometric, c. 24 nm in diameter (Peters, 1967; Takanami & Kubo, 1979a). Some particles seem to have small projections at the vertices (Fig. 6; I. M. Roberts & B. D. Harrison, unpublished results).
Particle Composition
Nucleic acid: RNA, single-stranded, c. 30% of particle weight. M. Wt c. 2.0 x 106, estimated by polyacrylamide gel electrophoresis under non-denaturing conditions. Sedimentation coefficient in 0.15 M sodium chloride, 0.015 M sodium citrate, pH 7.0 = 34.5 S (Rowhani & Stace-Smith, 1979; Takanami & Kubo, 1979b). Earlier reports (Sarkar, 1976) that the virus nucleic acid is DNA were not confirmed in more recent work. The virus RNA is covalently bonded to a genome-linked protein of M. Wt c. 7000 and contains no substantial polyadenylate sequence (Mayo et al., 1982).
Protein: c. 70% of particle weight. One main protein species of M. Wt c. 26,000, estimated by SDS/polyacrylamide gel electrophoresis (Rowhani & Stace-Smith, 1979).
Genome Properties
Virus particle RNA is infective, even after treatment with proteinase K, and is translated in rabbit reticulocyte lysates to give polypeptides of M. Wt c. 125,000 and 71,000, but not particle protein (Mayo et al., 1982). An additional translation product of M. Wt c. 29,000 is found with the wheat germ system but likewise does not react with antiserum to virus particles (Mayo & Barker, 1984). Nucleic acid extracts from potato leaves contain a subgenomic RNA species of M. Wt c. 1 x 106 in addition to molecules of M. Wt 2 x 106 (Barker, Mayo & Robinson, 1984).
Relations with Cells and Tissues
The virus is apparently confined to the phloem tissue of intact plants. In ultrastructural studies, the greatest numbers of virus particles are found in the cytoplasm of phloem parenchyma and companion cells, where they may form unstructured aggregates. Crystalline aggregates of virus particles are found in the vacuoles of some cells (Arai et al., 1969; Kojima et al., 1969). Vesicles occur in the cytoplasm and some of them seem to enter nuclei after fusing with the nuclear envelope (Shepardson, Esau & McCrum, 1980). Virus particle antigen can be detected in phloem cells by staining with fluorescent antibody (Weidemann & Casper, 1982) and variable amounts of necrosis develop in phloem tissue. Walls of primary phloem cells in stems and petioles become thickened. Callose accumulates in some sieve tubes of tubers and its presence is the basis of various staining tests (e.g. with 1% resorcin blue) used, before serological methods were developed, to assess the incidence of infection in stocks of seed tubers (De Bokx, 1967). Although mesophyll cells of intact leaves seem not to become infected, isolated mesophyll protoplasts of tobacco and potato can be infected by inoculation with purified virus in the presence of poly-L-ornithine (Kubo & Takanami, 1979; Barker & Harrison, 1982). Virus multiplication can be inhibited by adding actinomycin D to protoplast suspensions within 3 h after inoculation (Mayo & Barker, 1983).
Ecology and Control
In much of Western Europe, the virus seems essentially to be confined to potato, and infection is perpetuated in seed tubers, which give rise to infected plants. The virus is spread from these sources by winged and wingless aphids, principally Myzus persicae, to other potato plants, which are especially susceptible to infection when young. In some other countries, notably in S. America, Australia and, probably, in N. America, other solanaceous hosts occur and are likely to play a role as virus sources.
Control of the virus in potato crops relies mainly on:
1. Selecting tubers from symptom-free clones of mother plants.
2. Eliminating virus from individual tubers by heat treatment
(e.g. 10-20 days in air at 37.5°C; Kassanis,
1949).
3. Growing seed-potato crops in areas where vector aphids are
few or arrive late in the growing season, and
weather conditions are less favourable for aphid activity.
4. Roguing (removing) plants with symptoms of secondary infection
when the crop is young and before
vector aphids reach it.
5. Applying insecticides to crops to minimise aphid activity.
6. Harvesting the crop or destroying the haulms before recently
inoculated virus has passed from the shoots
to the tubers.
7. Isolating healthy seed-potato crops spatially from infected
crops.
8. Planting cultivars that are resistant to infection in field
conditions.
9. Assessing the health of tuber stocks by serological tests
(especially ELISA) after harvest.
Notes
Potato leafroll virus differs from most other viruses occurring in potato in possessing isometric particles c. 24 nm in diameter and in being transmissible in the persistent manner by Myzus persicae but not by inoculation with sap. It may not be easily distinguished from some other luteoviruses occurring in potato or tomato: indeed beet western yellows virus is reported to occur in leafroll-affected potato plants in the USA (Duffus, 1981a,1981b) and Tasmania (Duffus & Johnstone, 1982). Beet western yellows virus includes a range of strains, many of which (unlike potato leafroll virus) can infect Brassica spp. and Vicia faba, and/or cause obvious leaf yellowing in sugar beet. Some isolates of beet western yellows virus are serologically related to, though distinguishable from, isolates of potato leafroll virus; further work is needed with a large range of isolates to ascertain whether these two viruses can be reliably differentiated by serological tests. The degree of relationship between potato leafroll virus and luteovirus isolates obtained from tomato plants in the states of Florida (Zitter & Tsai, 1981) and Washington (Hassan & Thomas, 1981), USA, is not clear.
Figures

Potato (Solanum tuberosum ssp. tuberosum) cv. Up-to-Date plant with symptoms of secondary infection. (Courtesy J. A. T. Woodford).

Foliage of a Solanum tuberosum ssp. andigena clone, showing enanismo amarillo symptoms. (Courtesy R. A. C. Jones).

Experimentally infected plant of tomato cv. Grosse Lisse with yellow top disease. (Courtesy J. F. Thomas).

Physalis floridana plants infected with virulent (upper left), intermediate (upper right) and avirulent (lower left) virus strains. Uninfected plant (lower right). (Courtesy T. Tamada and B. D. Harrison).
References list for DPV: Potato leafroll virus (291)
- Altstatt & Ivanoff, Pl. Dis. Reptr 29: 29, 1945.
- Arai, Doi, Yora & Asuyama, Ann. phytopath. Soc. Japan 35: 10, 1969.
- Barker & Harrison, Plant Cell Reports 1: 247, 1982.
- Barker, Mayo & Robinson, Rep. Scott. Crop Res. Inst., 1983: 194, 1984.
- Braithwaite & Blake, Aust. J. agric. Res. 12: 1100, 1961.
- Casper, Phytopath. Z. 90: 364, 1977.
- Clarke, Converse & Kojima, Pl. Dis. 64: 43, 1980.
- Costa, Biologico 15: 79, 1949.
- Day, Aust. J. biol. Sci. 8: 498, 1955.
- De Bokx, Eur. Potato J. 10: 221, 1967.
- Duffus, Phytopathology 71: 193, 1981a.
- Duffus, Pl. Dis. 65: 819, 1981b.
- Duffus & Johnstone, Aust. J. exp. Agric. Anim. Husb. 22: 353, 1982.
- Eskandari, Sylvester & Richardson, Phytopathology 69: 45, 1979.
- Gildow, Phytopathology 72: 1289, 1982.
- Gugerli, Phytopath. Z. 96: 97, 1979.
- Gugerli, Potato Res. 23: 137, 1980.
- Harrison, Virology 6: 278, 1958a.
- Harrison, Virology 6: 265, 1958b.
- Hassan & Thomas, Phytopathology 71: 1004, 1981.
- Heinze, Phytopath. Z. 25: 103, 1955.
- Kassanis, Nature, Lond. 164: 881, 1949.
- Kennedy, Day & Eastop, A Conspectus of Aphids as Vectors of Plant Viruses, London, Commonwealth Institute of Entomology, 1962.
- Kojima, Bull. Fac. Agric. Niigata Univ. 33: 73, 1981.
- Kojima, Shikata, Sugawara & Murayama, Virology 39: 162, 1969.
- Kojima, Chou & Shikata, Ann. phytopath. Soc. Japan 44: 585, 1978.
- Kubo, CMI/AAB Descr. Pl. Viruses 234, 4 pp., 1981.
- Kubo & Takanami, Ann. phytopath. Soc. Japan 44: 398, 1978.
- Kubo & Takanami, J. gen. Virol. 42: 387, 1979.
- Maat & de Bokx, Neth. J. Pl. Path. 84: 149, 1978.
- Martin & Stace-Smith, Can. J. Pl. Path. 6: 206, 1984.
- Mayo & Barker, J. gen. Virol. 64: 1775, 1983.
- Mayo & Barker, Rep. Scott. Crop Res. Inst., 1983: 186, 1984.
- Mayo, Barker, Robinson, Tamada & Harrison, J. gen. Virol. 59: 163, 1982.
- Mehrad, Lapierre & Maury, C. r. hebd. Seanc. Acad. Sci., Paris D 286: 1179, 1978.
- Murayama & Kojima, Ann. phytopath. Soc. Japan 30: 209, 1965.
- Natti, Kirkpatrick & Ross, Am. Potato J. 30: 55, 1953.
- Peters, Virology 31: 46, 1967.
- Quanjer, Meded. LandbHoogesch. Wageningen 6: 41, 1913.
- Quanjer, Van der Lek & Oortwijn Botjes, Meded. LandbHoogesch. Wageningen 10: 1, 1916.
- Roberts & Harrison, Ann. appl. Biol. 93: 289, 1979.
- Roberts, Tamada & Harrison, J. gen. Virol. 47: 209, 1980.
- Rodriguez & Jones, Phytopathology 68: 39, 1978.
- Rowhani & Stace-Smith, Virology 98: 45, 1979.
- Sarkar, Virology 70: 265, 1976.
- Shepardson, Esau & McCrum, Virology 105: 379, 1980.
- Stegwee & Ponsen, Entomologia exp. appl. 1: 291, 1958.
- Sugawara, Kojima & Murayama, Ann. phytopath. Soc. Japan 39: 410, 1973.
- Sugawara, Kojima & Murayama, Ann. phytopath. Soc. Japan 40: 39, 1974.
- Sutton, Agric. Gaz. N.S.W. 66: 655, 1955.
- Takanami & Kubo, J. gen. Virol. 44: 153, 1979a.
- Takanami & Kubo, J. gen. Virol. 44: 853, 1979b.
- Tamada & Harrison, Ann. appl. Biol. 95: 209, 1980a.
- Tamada & Harrison, Ann. appl. Biol. 96: 67, 1980b.
- Tamada & Harrison, Ann. appl. Biol. 98: 261, 1981.
- Tamada, Harrison & Roberts, Ann. appl. Biol. 104: 107, 1984.
- Thomas, Ann. appl. Biol. 104: 79, 1984.
- Van Balen, Neth. J. Pl. Path. 88: 33, 1982.
- Waterhouse & Murant, Ann. appl. Biol. 97: 191, 1981.
- Webb, Larson & Walker, Am. Potato J. 28: 667, 1951.
- Webb, Larson & Walker, Res. Bull. Wis. agric. Exp. Stn 178, 1952.
- Weidemann, Z. angew. Ent. 94: 321, 1982.
- Weidemann & Casper, Potato Res. 25: 99, 1982.
- Zitter & Tsai, Pl. Dis. 65: 787, 1981.