Details of DPV and References
DPV NO: 293 July 1984
Family: Potyviridae
Genus: Potyvirus
Species: Watermelon mosaic virus | Acronym: WMV
This is a revised version of DPV 63
Watermelon mosaic virus 2
D. Purcifull Departments of Plant Pathology and Agronomy, University of Florida, Gainesville, Florida 32611, USA
E. Hiebert Departments of Plant Pathology and Agronomy, University of Florida, Gainesville, Florida 32611, USA
J. Edwardson Departments of Plant Pathology and Agronomy, University of Florida, Gainesville, Florida 32611, USA
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by Webb & Scott (1965) and Purcifull & Hiebert (1979). This virus was confounded in Description No. 63 with watermelon mosaic virus 1, which is now regarded as a strain of papaya ringspot virus).
Synonym
- General watermelon mosaic virus (Rev. appl. Mycol. 46: 818)
-
A virus with RNA-containing flexuous filamentous particles c. 760 nm long. It induces cylindrical (pinwheel) inclusions in the cytoplasm of host cells. It is readily mechanically transmissible, is transmissible by many species of aphid in a non-persistent manner and has a moderately wide host range. It causes diseases of various cucurbits, and is also found in natural infections of several leguminous and malvaceous species. Widely distributed throughout the world.
Main Diseases
Causes mosaic and mottle diseases of cantaloupe, cucumber, pumpkin, squash and watermelon. Reduces fruit production and quality in squash and other cucurbits (Thomas, 1971a; Greber, 1978; Demski & Sumner, 1979; Peña-Iglesias & Ayuso Gonzales, 1973). Also causes mottle diseases of pea (Inouye, 1964) and occurs naturally in various leguminous, malvaceous, and chenopodiaceous weeds, ornamentals and crop plants (Grogan, Hall & Kimble, 1959; Nelson & Tuttle, 1969; Adlerz, 1969).
Geographical Distribution
Reported in many areas of the world, including Australia (Greber, 1969), Czechoslovakia (Schmelzer & Milicic, 1966), Chile (Auger, Escaffi & Nome, 1974), France (Arteaga, Quiot & Leroux, 1976), Hungary (Horváth et al., 1975), Iran (Ebrahim-Nesbat, 1974), Israel (Russo et al., 1979), Italy (Lisa & Dellavalle, 1981), Japan (Yoshida et al., 1980), Mexico (Milne, Grogan & Kimble, 1969), New Zealand (Thomas, 1971a, 1971b), USA (Webb & Scott, 1965; Milne et al., 1969), Venezuela (Lastra, 1968) and Yugoslavia (Stakic & Nikolic, 1966).
Host Range and Symptomatology
Readily transmissible by mechanical inoculation. Over 160 dicotyledonous species in 23 families are susceptible (Molnar & Schmelzer, 1964; Edwardson, 1974).
-
Diagnostic species
- Cucurbita pepo
(pumpkin) cv. Small Sugar. Plants inoculated in the cotyledonary stage develop faint vein clearing symptoms in true leaves followed by systemic mottle (Fig. 1), mosaic (Fig. 2), and sometimes leaf distortion. - Nicotiana benthamiana. Mild mosaic and distortion of systemically infected
leaves. This species is susceptible to numerous isolates of watermelon mosaic virus 2,
but not susceptible to tested isolates of
papaya ringspot virus
(Christie & Crawford, 1978;
Purcifull & Hiebert, 1979;
Russo et al., 1979;
Purcifull et al., 1984b).
- Pisum sativum (pea) cvs. Alaska or Ranger. Mottle and necrosis in systemically infected leaves and stunting of plants. Some pea varieties (e.g. Bonneville) possess resistance, which is governed by a recessive gene (Schroeder & Provvidenti, 1971). Alaska pea is not susceptible to tested isolates of papaya ringspot virus (Purcifull & Hiebert, 1979; Russo et al., 1979; Purcifull et al., 1984b). Infection of inoculated leaves, however, was obtained with zucchini yellow mosaic virus (Purcifull et al., 1984a).
- Citrullus lanatus (watermelon). Systemic mosaic and leaf distortion (Fig. 3).
- Luffa acutangula. This species is not susceptible to most isolates of watermelon mosaic virus 2 although exceptions are reported (Milne et al., 1969). However, it is susceptible to isolates of papaya ringspot virus (Webb, 1965; Provvidenti & Schroeder, 1970; Greber, 1978; Purcifull & Hiebert, 1979; Russo et al., 1979; Purcifull et al., 1984b), and has been used to eliminate watermelon mosaic virus 2 from mixtures with papaya ringspot virus (Greber 1978; D. Purcifull, unpublished data). L. acutangula is also infected systemically by zucchini yellow mosaic virus (Lisa et al., 1981; Purcifull et al., 1984a).
- Pisum sativum (pea) cvs. Alaska or Ranger. Mottle and necrosis in systemically infected leaves and stunting of plants. Some pea varieties (e.g. Bonneville) possess resistance, which is governed by a recessive gene (Schroeder & Provvidenti, 1971). Alaska pea is not susceptible to tested isolates of papaya ringspot virus (Purcifull & Hiebert, 1979; Russo et al., 1979; Purcifull et al., 1984b). Infection of inoculated leaves, however, was obtained with zucchini yellow mosaic virus (Purcifull et al., 1984a).
-
Propagation species
- Cucurbita pepo (squash and pumpkin) is useful for maintaining cultures, for vector studies, and as a source of virus for purification (Purcifull & Hiebert, 1979). Nicotiana benthamiana also is a useful host for purification of virus particles and pinwheel inclusion body protein (Baum, 1980).
-
Assay species
- Chenopodium amaranticolor
forms well-defined lesions (Fig. 4) in response to infection by most isolates, but the virus is difficult to recover from the lesions by mechanical inoculation to cucurbits unless an inhibitor is removed by filtration through agarose columns (Milne et al., 1969). The virus also can be transmitted to cucurbits by aphids (Webb & Scott, 1965) or by using C. quinoa as an intermediate host (Greber, 1978). Most isolates induce local lesions also in C. quinoa, and some of them infect this species systemically (Rahimian & Izadpanah, 1978; Russo et al., 1979). These Chenopodium species are unreliable as diagnostic hosts for watermelon mosaic virus 2 because they also produce local lesions in response to zucchini yellow mosaic virus (Lisa et al., 1981; Purcifull et al., 1984a) and to some isolates of papaya ringspot virus (Purcifull & Hiebert, 1979; Russo et al., 1979; Yeh, Gonsalves & Provvidenti, 1984; Purcifull et al., 1984b).
Strains
Host range and symptom variants have been frequently reported (Grogan et al., 1959; Molnar & Schmelzer, 1964; Webb & Scott, 1965; Russo et al., 1979; Lisa & Dellavalle, 1981; Yamamoto, Ishii & Katsube, 1982a). Isolates from Italy, Australia, New Zealand and Israel have close serological affinities to isolates from the USA, as determined by immunodiffusion tests (Greber, 1978; Purcifull & Hiebert, 1979; Russo et al., 1979; Lisa & Dellavalle, 1981). Some isolates from Japan that have host range differences are serologically closely related, whereas other isolates are serologically distinguishable (Yamamoto et al., 1982a). A non-aphid-transmissible variant was reported in Europe (Molnar & Schmelzer, 1964). Resistance-breaking, so-called ‘thermal’, strains were obtained by maintaining resistant peas at 30°C after inoculation (Schroeder & Provvidenti, 1971).
Transmission by Vectors
Transmitted in a non-persistent manner by at least 38 species of aphid in 19 genera,
including Aphis citricola, A. craccivora, A. gossypii, Aulacorthum solani, Macrosiphum
euphorbiae, Myzus persicae and Toxoptera citricidus
(Karl & Schmelzer, 1971;
Adlerz, 1974;
Greber, 1978;
Yamamoto & Ishii, 1980;
Yamamoto et al.,1982b).
The virus was transmitted by M. persicae, A. citricola, A. craccivora
and A. gossypii following 10-60 s acquisition probes and inoculation access
periods of 1 h
(Adlerz, 1974).
A virus isolate maintained by sap transfer every 2-4 weeks
for 4 yr lost the ability to be aphid-transmitted
(Demski & Sumner, 1979).
The virus
was not aphid-transmissible from purified preparations by membrane feeding unless either
a soluble fraction from infected plants was added to the virus preparations, or the
aphids were allowed to feed on the soluble fraction before feeding on the virus
preparations
(Sako & Ogata, 1981).
This indicates that transmission of the virus
requires a helper factor, as reported for other
potyviruses
(Govier, Kassanis & Pirone, 1977).
Leafminer flies (Liriomyza sativae) inefficiently transmitted two isolates of the virus from squash to squash (Zitter & Tsai, 1977).
Transmission through Seed
No seed transmission was detected in the following species: cantaloupe (Grogan et al., 1959), cucumber (Inouye, 1964; Greber, 1969), patisson (Horváth et al., 1975), pea (Inouye, 1964), pumpkin (Greber, 1969), squash (Greber, 1969; Thomas, 1971a) or watermelon (Stakic & Nikolic, 1966).
Transmission by Dodder
Not transmitted by Cuscuta pentagona (Stakic & Nikolic, 1966).
Serology
Virus particles are a good immunogen. Sera with titres of 1/512 to 1/1024 have
been obtained
(Milne & Grogan, 1969;
Lisa & Dellavalle, 1981).
Immunoprecipitin tests in liquid have been used to study relationships
(van Regenmortel, Brandes & Bercks, 1962;
Webb & Scott, 1965;
Milne & Grogan, 1969;
Lisa & Dellavalle, 1981).
Immunodiffusion tests detect the virus in crude extracts if sodium dodecyl sulphate
(SDS) is added to the agar medium (0.8% purified agar, 0.5% SDS, 1.0% sodium azide)
and to crude sap (1 g tissue: 1 ml water: 1 ml 3% SDS) to dissociate the virus into
diffusible fragments
(Fig. 8)
(Purcifull & Batchelor, 1977;
Purcifull & Hiebert, 1979).
SDS-immunodiffusion tests have been used extensively for studying
relationships of the virus
(Purcifull & Hiebert, 1979;
Russo et al., 1979;
Baum, Purcifull & Hiebert, 1979;
Lisa et al., 1981;
Lisa & Dellavalle, 1981;
Yamamoto et al., 1982a).
A modification of the procedure permits
use of the same extracts for infectivity and SDS-immunodiffusion tests
(Lecoq & Lot, 1982).
Other materials that dissociate the virus into diffusible components
include ethanolamine
(Uyemoto, Provvidenti & Purcifull, 1973)
and pyrrolidine
(Shepard, Secor & Purcifull, 1974).
The double-antibody sandwich form of the enzyme-linked immunosorbent assay (ELISA)
(Clark & Adams, 1977)
enabled detection of the virus at concentrations of 25-50
ng/ml in purified preparations or at crude sap dilutions of 10-4 to
10-5
(Sako, Matsuo & Nonaka, 1980a,
1980b,
1982).
ELISA was also used to study virus relationships
(Baum, 1980).
Relationships of the virus to other
potyviruses
have also been studied by immunoelectron microscopic procedures
(Makkouk & Lesemann, 1980;
Lisa et al., 1981;
Samah, 1982).
Freeze-dried extracts and leaf discs desiccated over calcium chloride have been
used as reference antigens for SDS-immunodiffusion
(Purcifull & Hiebert, 1979)
and ELISA
(Sako et al., 1980b),
respectively. SDS-immunodiffusion
tests are also useful for studying relationships of pinwheel inclusion proteins
(Baum, 1980;
Baum & Purcifull, 1981;
Yeh, 1984).
Indirect ELISA also has been
used for this purpose
(Yeh, 1984).
Immunoprecipitation techniques with antisera to the virus particle protein, to the inclusion-body protein and to tobacco vein mottling virus helper component protein have been used to analyse the in vitro translation products of watermelon mosaic virus 2 RNA (Hiebert, 1981; Hiebert, Thornbury & Pirone, 1984b).
Relationships
The virus is classified as a member of the
potyvirus group,
on the basis of its particle morphology, serological relationships to
potato virus Y
and other potyviruses, aphid transmissibility, and ability to induce pinwheel
inclusions in host cells
(Hollings & Brunt, 1981;
Matthews, 1982).
Edwardson (1974)
assigned it to his Subdivision III of the potyvirus group.
The relationships and the nomenclature of certain potyviruses that affect cucurbits have
been controversial
(van Regenmortel, 1971;
Lovisolo, 1980).
Watermelon mosaic virus was divided into two groups of strains, 1 and 2, by
Webb & Scott (1965),
on the basis of a lack of serological relatedness and on major differences in host range, but
Milne & Grogan (1969)
concluded that members of groups 1 and 2 were closely related serologically
and should be regarded as strains of the same virus. However, more recent work with
isolates from the USA, Europe, Australia and the Mediterranean area indicates that there
are significant serological
(Fig. 8),
host range, and cytological distinctions between the two groups
(Edwardson, 1974;
Christie & Edwardson, 1977;
Purcifull & Hiebert, 1979;
Russo et al., 1979),
although distant serological relationships have been detected in some cases
(Purcifull & Hiebert, 1979,
and unpublished data;
Makkouk & Lesemann, 1980;
Samah, 1982;
Dodds et al., 1984).
Moreover, isolates of group 1 lack nucleic acid homology with isolates of group 2
(Samah, 1982).
Webb & Scott’s watermelon mosaic virus 2 is the subject of this Description. Their watermelon
mosaic virus 1 is now regarded as a strain (type W) of
papaya ringspot virus.
The degree of antigenic variation among isolates of watermelon mosaic virus 2 needs
further evaluation. Although close serological relationships were found in
SDS-immunodiffusion tests between isolates from the USA (Florida, New York, California,
Arizona), Italy, Israel, Australia and New Zealand
(Greber, 1978;
Purcifull & Hiebert, 1979;
Russo et al., 1979;
Lisa & Dellavalle, 1981),
serologically distinguishable strains of watermelon mosaic virus 2 have been reported in Japan
(Yamamoto et al., 1982a)
and Jordan
(Al-Musa & Mansour, 1982)
(also based on SDS-immunodiffusion).
Zucchini yellow mosaic virus
is serologically related to, but distinct from, watermelon mosaic virus 2
(Lisa et al., 1981;
Lisa & Lecoq, 1984;
Purcifull et al., 1984a)
(Fig. 8).
Bean yellow mosaic virus, some isolates of which infect cucurbits
(Provvidenti & Uyemoto, 1973),
also is serologically related to watermelon mosaic virus 2
(Lisa & Dellavalle, 1981).
A potyvirus isolated from cucurbits in Morocco was identified as a strain of watermelon
mosaic virus 2 primarily on the basis of certain host reactions
(Fischer & Lockhart, 1974).
However, other host reactions
(Purcifull & Hiebert, 1979),
serological tests with virus particle proteins
(Purcifull & Hiebert, 1979;
Baum et al., 1979;
Baum, 1980),
peptide mapping of the particle protein
(Baum, 1980)
and serological relationships of cylindrical inclusion protein
(Baum, 1980;
Baum & Purcifull, 1981),
all suggest that the two viruses are distinct.
Immunodiffusion tests indicate that the virus particle protein of watermelon mosaic virus 2 is related to, but distinct from, those of bean common mosaic, blackeye cowpea mosaic, soybean mosaic, tobacco etch and potato Y viruses (Uyemoto et al., 1973; Shepard et al., 1974; Purcifull & Hiebert, 1979; Lima et al., 1979; Baum, 1980).
Stability in Sap
Three of seven isolates compared by Webb & Scott (1965) were inactivated by heating to 60°C but not to 55°C for 10 min; the other four isolates were inactivated by heating to 65°C. Some isolates lost infectivity after 10-20 days’ storage at 18-24°C whereas others were infective after 50 days but lost infectivity after 60 days. All isolates were infective after dilution to 10-2; others withstood dilution to 10-4, but were non-infective after dilution to 5 x 10-4.
Purification
The virus particles and the cylindrical inclusions induced in infected tissues have
both been purified.
Virus particle purification. [Modification of procedure used by
Purcifull & Hiebert (1979)].
Homogenise infected pumpkin (Small Sugar) tissue (100 g), collected 3-4 wk
after inoculation, in a mixture of 200 ml 0.5 M potassium phosphate buffer (PB), pH 7.5,
containing 0.2% sodium sulphite, 10 mM EDTA and 24 ml n-butanol. Stir for 2 h at 4°C.
Centrifuge at 10,000 g for 15 min. To the supernatant fluid add Triton
X-100 to 1% (v/v), polyethylene glycol (PEG, M. Wt 6000) to 6% (w/v) and NaCl to 100 mM
and stir for 1 h at 4°C. Centrifuge at 10,000 g for 10 min and discard
the supernatant fluid. Resuspend the pellets in 30 ml 50 mM PB (pH 8.2), containing 10 mM
EDTA, with the aid of a glass tissue grinder. Centrifuge at 10,000 g for 10
min and discard the pellet. Re-precipitate the virus particles from the supernatant fluid
by adding PEG to 8% (w/v) and NaCl to 100 mM and stir for 30 min. Centrifuge at 27,000 g for 10 min. Resuspend the pellets in 2-4 ml 50 mM PB (pH 8.2), containing
10 mM EDTA, with the aid of a tissue grinder. Layer the resuspended material onto 30% CsCl
(w/w) in 50 mM PB (pH 8.2) containing 10 mM EDTA and centrifuge at 140,000 g
for 16-18 h at 5°C. Collect the virus-containing zone by droplet fractionation, dilute
with an equal volume of buffer and then centrifuge at 12,000 g for 10 min.
Recover the virus from the supernatant fluid by PEG precipitation as before. The yield of
virus particles is about 2-5 mg/100 g tissue.
Cylindrical inclusion purification. [Modification of procedures outlined by Lima et al. (1979), and by Hiebert, Purcifull & Christie (1984a)]. To purify cylindrical (pinwheel) inclusions collect systemically infected Small Sugar pumpkin tissue (3-4 wk after inoculation) and homogenise 100 g tissue in 200 ml 0.5 M PB (pH 7.5) containing 10 mM EDTA and 0.1% sodium sulphite, and 100 ml of a 1:1 mixture of chloroform and carbon tetrachloride. Break the emulsion by centrifugation at 1000 g for 5 min. Retain the aqueous phase and re-extract the pellet with PB as above and centrifuge again. Combine the supernatant fluid with the aqueous phase from the previous centrifugation and centrifuge at 13,000 g for 15 min. (The supernatant fluid from this centrifugation may be processed for virus purification as described above.) Resuspend the pellets in 50 ml of 50 mM PB (pH 8.2) containing 10 mM EDTA and 0.1% mercaptoethanol. Add Triton X- 100 to 5% (v/v), stir for 1 h and centrifuge the mixture at 27,000 g for 15 min. Resuspend the pellets in 25 ml of 50 mM PB (pH 8.2) containing 10 mM EDTA and 0.1% 2-mercaptoethanol. Centrifuge at 27,000 g for 15 min. Resuspend the pellets in 5-10 ml buffer as above with the aid of a Sorvall Omnimixer. Layer the resuspended material onto a sucrose step gradient (50, 60 and 80%, w/w) and centrifuge at 70,000 g in a Beckman SW 25.1 rotor for 1 h. Recover the inclusions that collect on top of the 60 and 80% sucrose zones. Dilute these fractions four-fold and centrifuge at 27,000 g for 15 min to sediment the inclusions. Yields up to 10 A280 units per 100 g tissue are obtained.
Properties of Particles
A260/A280: 1.2 (Baum, 1980).
Particle Structure
Flexuous filaments (Fig. 5), typical of potyviruses, c. 750-780 nm long (Schmelzer, 1966; Purcifull, 1968; Milne & Grogan, 1969; Russo et al., 1979).
Particle Composition
Nucleic acid: RNA (Samah, 1982). Isolation by the method of Brakke & van Pelt (1970), in 100 mM ammonium carbonate, pH 9.0, containing 1 mM EDTA and 1% SDS, yields RNA that sediments at 39 S, like that of other potyviruses (E. Hiebert, unpublished data).
Protein: Subunit M. Wt 3.4 x 104, but most preparations also contain a protein of M. Wt 2.9-2.95 x 104 (Baum, 1980; Vovlas, Hiebert & Russo, 1981), which may represent a proteolytic degradation product as detected with other potyviruses (Hiebert & McDonald, 1973).
Genome Properties
Watermelon mosaic virus 2 RNA showed no homology in hybridisation analysis with complementary DNA prepared to a type W isolate of papaya ringspot virus (Samah, 1982). The RNA is translated efficiently in vitro in rabbit reticulocyte lysates (Hiebert, 1981). The in vitro translation product profile is similar to that obtained for tobacco etch and pepper mottle viruses (Dougherty & Hiebert, 1980). A proposed genetic map is illustrated in Fig. 9. The major product of RNA translation in vitro, mapped at the 5' end, appears unrelated to a corresponding product of a type W isolate of papaya ringspot virus, when these products are compared by peptide mapping (E. Hiebert, unpublished data). Both products, however, are precipitated by antiserum to the helper component protein of tobacco vein mottling virus (Hiebert et al., 1984b).
Relations with Cells and Tissues
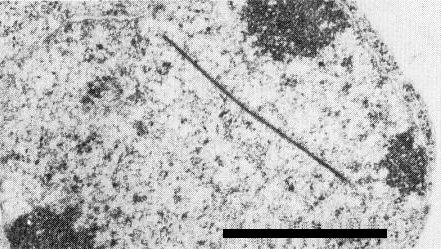
Cylindrical inclusions (which appear as pinwheels in transverse sections) and scrolls have been detected in the cytoplasm of all isolates studied (Edwardson, 1966, 1974; Christie & Edwardson, 1977; Russo et al., 1979) (Fig. 7). Laminated aggregates (Fig. 7) also occur, with some isolates at least, from the USA (Florida, California, Arizona) and New Zealand (J. Edwardson, unpublished data), and from Australia (Greber, 1978). The laminated aggregates are finely striated, are severely disrupted in the presence of potassium phosphotungstate, and are more readily preserved for electron microscopy by mounting in ammonium molybdate (Purcifull, 1968) or uranyl acetate (Hiebert & McDonald, 1973; Baum, 1980). The protein subunit of the cylindrical inclusion has a M. Wt of 6.9 x 104 and is serologically distinct from the virus particle protein and from host protein (Baum, 1980). Thin, plate-like inclusions are observed by light and electron microscopy in the nuclei of cells infected with the Florida and Arizona isolates (Christie & Edwardson, 1977) (Fig. 6). Cytoplasmic inclusions (cylindrical inclusions and associated structures) and nuclear inclusions are both detectable by light microscopy of epidermal cells stained with luxol brilliant green-calcomine orange (Christie & Edwardson, 1977). The cytoplasmic, amorphous (irregular) inclusions that are characteristic of papaya ringspot virus (Christie & Edwardson, 1977; Russo et al., 1979; Purcifull et al. 1984b) are not detected in cells infected with watermelon mosaic virus 2 (Edwardson, 1974; Christie & Edwardson, 1977; Russo et al., 1979). Large, organised aggregates of filamentous particles associated with membrane-like components have been observed in negatively stained leaf extracts (Purcifull, Edwardson & Christie, 1968).
Notes
It is not possible at present to determine the status of some of the viruses that have
been named watermelon mosaic virus, including original isolates in Florida
(Anderson, 1954)
and certain South African isolates
(van Regenmortel et al., 1962).
Differences in serological reactions
(Webb & Scott, 1965)
and host reactions have led some workers to
consider the South African isolates as distinct
(Schmelzer, 1969;
Horváth et al., 1975).
The amorphous cytoplasmic inclusions that typically occur with type W isolates of
papaya ringspot virus
aid the distinction of this virus from watermelon mosaic virus 2
(Edwardson, 1974;
Christie & Edwardson, 1977;
Russo et al., 1979).
Papaya ringspot virus and
watermelon mosaic virus 2 also can be distinguished by SDS-immunodiffusion tests
(Purcifull & Hiebert, 1979;
Russo et al., 1979;
Gonsalves & Ishii, 1980).
Several other
potyviruses
that affect cucurbits in nature also can be distinguished from watermelon mosaic
virus 2 by SDS-immunodiffusion tests, including
zucchini yellow mosaic,
bean yellow mosaic,
zucchini yellow fleck, and a potyvirus from Morocco
(Purcifull & Hiebert, 1979;
Vovlas et al., 1981;
Lisa et al., 1981;
Baum et al.,1979).
Many other viruses affect cucurbits (Lovisolo, 1980). Two that occur in various areas of the world are cucumber mosaic and squash mosaic viruses. They can be distinguished from watermelon mosaic virus 2 by their isometric particle morphologies (Francki, Mossop & Hatta, 1979; Campbell, 1971), by host reactions (Grogan et al., 1959, Milne et al., 1969), by the morphology of the inclusion bodies detected by light microscopy (Christie & Edwardson, 1977), and by SDS-immunodiffusion tests (Purcifull, Christie & Lima, 1981; Purcifull et al., 1984a).
Figures

Flexuous particles in leaf extract, negatively stained with potassium phosphotungstate. Bar represents 500 nm.

Cytoplasmic inclusions in infected cell. Cylindrical pinwheel inclusion (ci); scroll (s); and laminated aggregate (la). Bar represents 0.5 µm.

Sodium dodecyl sulphate (SDS)-immunodiffusion tests with SDS-treated squash (Cucurbita pepo) leaf extracts. 1 = type W isolate of papaya ringspot virus; 2 = watermelon mosaic virus 2; 3 = zucchini yellow mosaic virus; 4 = healthy; 5 = squash mosaic virus; 6 = cucumber mosaic virus. A = antiserum to watermelon mosaic virus 2, collected 1 month after immunisation of a rabbit; B = antiserum from the same rabbit, 10 months after immunisation; C = antiserum as in B, except that intragel absorption was accomplished by adding sap from zucchini yellow mosaic virus-infected leaves to the centre well c. 16 h prior to adding antiserum.

Proposed genetic map for watermelon mosaic vius 2. The M. Wt of the gene products are presented above the map, and two products are identified below the map. The 107k product is serologically related to tobacco vein mottling virus helper component protein, and the 49k and 54k products are serologically related to tobacco etch virus nuclear inclusion proteins.
References list for DPV: Watermelon mosaic virus 2 (293)
- Adlerz, Proc. Fla St. hort. Soc. 82: 161, 1969.
- Adlerz, Phytopathology 64: 350, 1974.
- Al-Musa & Mansour, Pl. Dis. 66: 330, 1982.
- Anderson, Phytopathology 44: 198, 1954.
- Arteaga, Quiot & Leroux, Annls Phytopath. 8: 347, 1976.
- Auger, Escaffi & Nome, Pl. Dis. Reptr 58: 599, 1974.
- Baum, Ph.D. Diss., Univ. Fla, 95 pp., 1980.
- Baum & Purcifull, Phytopathology 71: 202, 1981.
- Baum, Purcifull & Hiebert, Phytopathology 69: 1023, 1979.
- Brakke & Van Pelt, Virology 42: 699, 1970.
- Campbell, CMI/AAB Descr. Pl. Viruses 43, 4 pp., 1971.
- Christie & Crawford, Pl. Dis. Reptr 62: 20, 1978.
- Christie & Edwardson, Monogr. Ser. Fla agric. Exp. Stn 9, 155 pp., 1977.
- Clark & Adams, J. gen. Virol. 34: 475, 1977.
- Demski & Sumner, Res. Bull. Ga agric. Exp. Stn 234, 15 pp., 1979.
- Dodds, Lee, Nameth & Laemmlen, Phytopathology 74: 221, 1984.
- Dougherty & Hiebert, Virology 104: 183, 1980.
- Ebrahim-Nesbat, Phytopath. Z. 79: 352, 1974.
- Edwardson, Am. J. Bot. 53: 359, 1966.
- Edwardson, Monogr. Ser. Fla agric. Exp. Stn 4, 398 pp., 1974.
- Fischer & Lockhart, Pl. Dis. Reptr 58: 143, 1974.
- Francki, Mossop & Hatta, CMI/AAB Descr. Pl. Viruses 213, 6 pp., 1979.
- Gonsalves & Ishii, Phytopathology 70: 1020, 1980.
- Govier, Kassanis & Pirone, Virology 78: 306, 1977.
- Greber, Qd J. agric. Anim. Sci. 26: 145, 1969.
- Greber, Aust. J. agric. Res. 29: 1235, 1978.
- Grogan, Hall & Kimble. Phytopathology 49: 366, 1959.
- Hiebert, Abstr. 5th int. Congr. Virol., 1981: 66, 1981.
- Hiebert & McDonald, Virology 56: 349, 1973.
- Hiebert, Purcifull & Christie, in Methods in Virology, Vol. 8, pp. 225-280, eds K. Maramorosch & H. Koprowski, New York, Academic Press, 1984a.
- Hiebert, Thornbury & Pirone, Virology 135: 1, 1984b.
- Hollings & Brunt, CMI/AAB Descr. Pl. Viruses 245, 7 pp., 1981.
- Horváth, Juretic, Besada & Kuroli, Acta phytopath. Acad. Sci. hung. 10: 93, 1975.
- Inouye, Ber. Ohara Inst. landw. Biol. 12: 133, 1964.
- Karl & Schmelzer, Arch. PflSchutz 7: 3, 1971.
- Lastra, Pl. Dis. Reptr 52: 171, 1968.
- Lecoq & Lot, Agronomie 2: 786, 1982.
- Lima, Purcifull & Hiebert, Phytopathology 69: 1252, 1979.
- Lisa & Dellavalle, Phytopath. Z. 100: 279, 1981.
- Lisa & Lecoq, CMI/AAB Descr. Pl. Viruses 282, 4 pp., 1984.
- Lisa, Boccardo, D’Agostino, Dellavalle & d’Aquilio, Phytopathology 71: 667, 1981.
- Lovisolo, Acta Hort. 88: 33, 1980.
- Makkouk & Lesemann, Pl. Dis. 64: 799, 1980.
- Matthews, Intervirology 17: 4, 1982.
- Milne & Grogan, Phytopathology 59: 809, 1969.
- Milne, Grogan & Kimble, Phytopathology 59: 819, 1969.
- Molnar & Schmelzer, Phytopath. Z. 51: 361, 1964.
- Nelson & Tuttle, Phytopathology 58: 849, 1969.
- Peña-Iglesias & Ayuso Gonzalez, Monogr. Minist. Agric. Madrid 20, 121 pp., 1973.
- Provvidenti & Schroeder, Pl. Dis. Reptr 54: 744, 1970.
- Provvidenti & Uyemoto, Pl. Dis. Reptr 57: 280, 1973.
- Purcifull, Virology 36: 690, 1968.
- Purcifull & Batchelor, Bull. Fla agric. Exp. Stn 788, 39 pp., 1977.
- Purcifull & Hiebert, Phytopathology 69: 112, 1979.
- Purcifull, Edwardson & Christie, Virology 35: 478, 1968.
- Purcifull, Christie & Lima, Phytopathology 71: 1221, 1981.
- Purcifull, Adlerz, Simone, Hiebert & Christie, Pl. Dis. 68: 230, 1984a.
- Purcifull, Edwardson, Hiebert & Gonsalves, CMI/AAB Descr. Pl. Viruses 292, 8 pp., 1984b.
- Rahimian & Izadpanah, Phytopath. Z. 92: 305, 1978.
- Russo, Martelli, Vovlas & Ragozzino, Phytopath. Medit. 18: 94, 1979.
- Sako & Ogata, Virology 112: 762, 1981.
- Sako, Matsuo & Nonaka, Ann. phytopath. Soc. Japan 46: 639, 1980a.
- Sako, Matsuo & Nonaka, Ann. phytopath. Soc. Japan 46: 647, 1980b.
- Sako, Matsuo & Nonaka, Ann. phytopath. Soc. Japan 48: 192, 1982.
- Samah, Ph.D. Diss., Univ. Adelaide, 109 pp., 1982.
- Schmelzer, Naturwissenschaften 53: 619, 1966.
- Schmelzer, in Plant Virology, p. 206, ed. C. Blattny, Academia: Prague, 346 pp., 1969.
- Schmelzer & Milicic, Phytopath. Z. 57: 8, 1966.
- Schroeder & Provvidenti, Phytopathology 61: 846, 1971.
- Shepard, Secor & Purcifull, Virology 58: 464, 1974.
- Stakic & Nikolic, Savr. Poljopr. 3: 289, 1966.
- Thomas, N. Z. Jl agric. Res. 14: 235, 1971a.
- Thomas, N. Z. Jl agric. Res. 14: 242, 1971b.
- Uyemoto, Provvidenti & Purcifull, Phytopathology 63: 208, 1973.
- Van Regenmortel, CMI/AAB Descr. Pl. Viruses 63, 4 pp., 1971.
- Van Regenmortel, Brandes & Bercks, Phytopath Z. 45: 205, 1962.
- Vovlas, Hiebert & Russo, Phytopath. Medit. 20: 123, 1981.
- Webb, Phytopathology 55: 1379, 1965.
- Webb & Scott, Phytopathology 55: 895, 1965.
- Yamamoto & Ishii, Proc. Ass. Pl. Prot. Shikoku 15: 37, 1980.
- Yamamoto, Ishii & Katsube, Ann. phytopath. Soc. Japan 48: 613, 1982a.
- Yamamoto, Ishii, Katsube & Sorin, Jap. J. appl. Ent. Zool. 26: 218, 1982b.
- Yeh, Ph.D. Diss., Cornell Univ., 98 pp., 1984.
- Yeh, Gonsalves & Provvidenti, Phytopathology 74: 1081, 1984.
- Yoshida, Goto, Nemoto & Tsuchizaki, Ann. phytopath. Soc. Japan. 46: 339, 1980.
- Zitter & Tsai, Pl. Dis. Reptr 61: 1025, 1977.