Details of DPV and References
DPV NO: 296 September 1985
Family: Reoviridae
Genus: Phytoreovirus
Species: Rice gall dwarf virus | Acronym: RGDV
Rice gall dwarf virus
T. Omura Laboratory of Virus Disease Control, National Agriculture Research Center, Tsukuba Science City, Yatabe, Ibaraki 305, Japan
H. Inoue Laboratory of Insect Pest Forecasting, Kyushu National Agricultural Experiment Station, Chikugo, Fukuoka 833, Japan
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
First described by Putta et al. (1980) and Omura et al. (1980).
A virus with polyhedral particles c. 65 nm in diameter, containing double-stranded RNA in 12 segments. Transmitted in the persistent manner and transovarially by leafhoppers (Delphacidae). Infects species of Gramineae. Not mechanically transmissible to plants. Causes severe and economically important disease in rice. Occurs in China and South-East Asia.
Main Diseases
Field-infected plants of rice (Oryza sativa) cv. Taichung Native 1 are stunted (Fig. 1), with small whitish galls (Fig. 2) mostly less than 2 mm long and 0.4-0.5 mm wide along the under sides of the leaf blades and outer sides of the leaf sheaths (Putta et al., 1980; Omura et al., 1980). Infected plants turn darker green than normal and remain green when mature, whereas healthy plants turn yellow. Severe dwarfing, delayed flowering, incomplete panicle emergence and unfilled grains lead to loss of yield which in the most seriously affected fields may be up to 4500 kg/ha (Faan et al., 1983).
Geographical Distribution
Reported from Thailand, Malaysia and China (Putta et al., 1980; Omura et al., 1980; Ong & Omura 1982; Faan et al., 1983).
Host Range and Symptomatology
Only Oryza sativa is infected naturally (Putta et al., 1980; Omura et al., 1980; Ong & Omura, 1982; Faan et al., 1983). Using viruliferous insects, Putta et al. (1982) experimentally infected wild rice (Oryza rufipagon), barley, wheat, rye, oat, Italian ryegrass and Alopecurus aequalis. The symptoms in these plants resemble those occurring in rice. The leafhopper vector is a host, but no disease is reported in virus-carrying insects.
-
Diagnostic species
- Oryza sativa
(rice), cv. Taichung Native 1. Stunting and formation of leaf galls as described under Main Disease.Propagation species
- The virus can be propagated in rice inoculated by means of viruliferous insects.
Assay species
- Young rice seedlings are suitable for assaying transmission by insect vectors. Monolayers of insect vector cells proved to be an excellent assay system for the virus (Omura et al., 1982a).
Strains
None reported.
Transmission by Vectors
The vectors are leafhoppers (delphacids) in the genera Nephotettix and Recilia (Inoue & Omura, 1982; Morinaka et al., 1982). The proportion of transmitting individuals differs considerably among species and colonies: 1.8-95.0% for N. nigropictus (Fig. 3), 1.4-42.7% for N. cincticeps, 9.4% for N. malayanus, 0.1-0.7% for N. virescens and 4.1-17.1% for R. dorsalis (Inoue & Omura, 1982; Morinaka et al., 1982). The virus is transmitted by vectors in a persistent manner and the daily transmission pattern is intermittent. The minimum acquisition access and minimum inoculation access periods for R. dorsalis are 8 h and 1 h, respectively, at 28-34°C (Morinaka et al., 1982). The average incubation period at 25°C is c. 14.5 days for N. nigropictus, N. cincticeps and N. malayanus, and 17-18 days for N. virescens and R. dorsalis, whereas it is c. 8 days for R. dorsalis and N. nigropictus at 28-34°C (Inoue & Omura, 1982; Morinaka et al., 1982). The virus is transmitted from infective females to their progeny through the eggs: the percentage of congenitally infective progeny among broods of N. nigropictus ranges from 0-100% (Inoue & Omura, 1982; Morinaka et al., 1982). The virus multiplies in the leafhoppers (Omura et al., 1984). The planthoppers, Nilaparvata lugens and Laodelphax striatellus, did not transmit the virus (Inoue & Omura, 1982).
Transmission through Seed
None found (Morinaka et al., 1982).
Serology
Omura et al. (1982b) obtained rabbit antiserum with a precipitin ring interface titre of 1/2048 against virus particles; it also had a titre of 1/2 against rice gall dwarf virus dsRNA, rice dwarf virus dsRNA and poly(I):poly(C). The antiserum was used to detect virus antigen in plant and leafhopper extracts by the latex flocculation test and by enzyme-linked immunosorbent assay (Omura et al., 1984). Immunosorbent electron microscopy and antibody-coating tests are also effective (Boccardo et al., 1984).
Relationships
The particle morphology (double-shelled particles about 65 nm in diameter) suggests that the virus belongs to the plant reovirus group (Omura et al., 1982b). Various other properties suggest that it should be placed in the phytoreovirus sub-group rather than the fijivirus sub-group. The virus particles were stable after chloroform and heat treatments, repeated freezing and thawing, and staining with phosphotungstate (Omura et al., 1982b), all of which degrade the outer shell of the particles of fijiviruses to give spiked and smooth cores, but do not damage the particles of phytoreoviruses (Milne & Lovisolo, 1977). The genome resembles those of the phytoreoviruses in the total amount of dsRNA and in its division into 12 segments (Hibi et al., 1984; Boccardo et al., 1984). Transmission by leafhoppers but not planthoppers, and the high frequency of transovarial transmission (Inoue & Omura, 1982) also suggest that the virus belongs to the phytoreovirus sub-group (Milne & Lovisolo, 1977; Fenner & Gibbs, 1983).
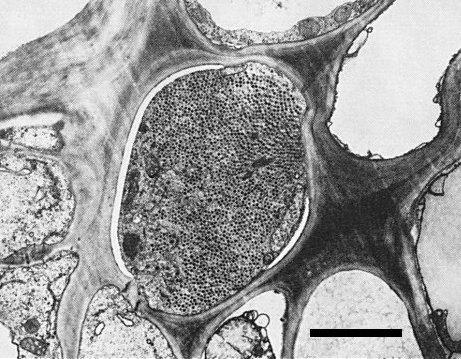
Though rice gall dwarf virus and rice dwarf virus have particles with similar morphology (Iida et al., 1972; Omura et al., 1982b; Boccardo et al., 1984), are transmitted in the same manner by leafhoppers (Inoue & Omura, 1982; Morinaka et al., 1982; Iida et al., 1972) and have a common host range (Putta et al., 1982; Iida et al., 1972), they are readily distinguishable by leaf symptoms (see Notes). Also, rice gall dwarf virus is restricted to phloem parenchyma cells (Fig. 6) whereas rice dwarf virus is found in many kinds of cell in infected rice plants (Iida et al., 1972). No serological relationship was observed between the two viruses (Omura et al., 1982b). The M. Wt of the nucleic acid species (Fig. 4) and particle proteins (Omura et al., 1985) of both viruses are also distinct. No sequence homology was found between rice dwarf virus RNA and RNA transcribed from rice gall dwarf virus (Yokoyama et al., 1984).
Stability in Sap
Extracts of virus-infected leaves, assayed by inoculating cell monolayers of vector insects, were infective at dilutions of 10-7; a sample diluted to 10-4 lost infectivity after being heated to 60°C for 10 min (Omura et al., 1982a).
Purification
(Omura et al., 1982b). Harvest rice leaves or roots about 40 days after inoculation of the plants, and grind each 100 g with a meat grinder in 200 ml 0.1 M sodium phosphate buffer, pH 7.0, containing 0.01 M MgCl2 (added immediately before use). Pass the homogenate through a layer of fine cotton cloth, mix the filtrate with CCl4 to 20% (v/v), and blend in a Waring Blendor for 2 min. Centrifuge for 15 min at 3000 g, and mix the supernatant fluid with polyethylene glycol (M. Wt 6000), NaCl and Triton X-100 to final concentrations of 6% (w/v), 0.3 M and 1% (w/v), respectively. Stir the mixture for 40 min and centrifuge for 15 min at 6000 g. Resuspend the pellet in 0.1 M histidine buffer, pH 7.0, containing 0.01 M MgCl2 (His-Mg), incubate for 30 min and centrifuge for 15 min at 3000 g. To the supernatant fluid add CCl4 to 10% (v/v) and centrifuge for 40 min at 96,000 g. Resuspend the pellet in His-Mg, incubate for 30 min, and centrifuge for 15 min at 3000 g. Layer the supernatant fluid on a 10-40% (w/v) linear sucrose gradient in His-Mg and centrifuge for 1 h at 87,000 g. Recover the virus-containing zone, layer on a 40-60% (w/v) linear sucrose gradient in His-Mg, and centrifuge for 15 h at 62,000 g. Recover the virus-containing zone and concentrate the virus particles by centrifugation for 2 h at 96,000 g. The yield of purified virus is c. 1 mg/kg root and c. 0.25 mg/kg leaf.
Properties of Particles
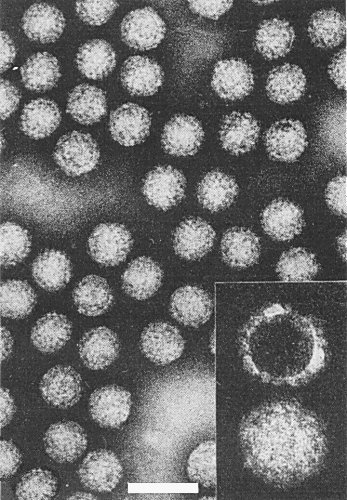
The particles are resistant to organic solvents (Omura et al., 1982b) and appear completely double-shelled in purified preparations (Fig. 5). The outer shell of the particles was removed by treatment with CsCl in 0.1 M His-Mg buffer at pH > 7.5 (Omura et al., 1985).
A260/A280: 1.42 for intact virus particles and 1.62 for core particles (Omura et al., 1985).
Particle Structure
Purified particles negatively stained with phosphotungstate, uranyl acetate or ammonium molybdate are polyhedral and about 65 nm in diameter (Omura et al., 1982b; Boccardo et al., 1984); particles composed of only an inner core are not found nor are there any surface projections like those found on the particles of fijiviruses (Milne & Lovisolo, 1977). Phosphotungstate does not damage the particles as it does those of rice ragged stunt virus (Omura et al., 1983; Boccardo & Milne, 1984) and the particles remain undamaged by treatment with chloroform, by repeated freezing and thawing, or by treatment for 10 min at 50°C (Omura et al., 1982b).
Particle Composition
Nucleic acid: RNA, double-stranded (Hibi et al., 1984). The melting transition temperature in 1.5 mM NaCl, 0.15 mM Na citrate, pH 7.0, is 76.2°C and the hyperchromicity is 30.6%. Buoyant density is 1.596 g/cm3 in Cs2SO4. The RNA consists of 12 segments in equimolar amounts (Fig. 4) (Hibi et al., 1984; Boccardo et al., 1984). The total M. Wt (x 10-6) of the RNA has been estimated as 16.92, with segments of 3.10, 2.48, 2.25, 1.90, 1.82, 1.11, 1.11, 0.96, 0.68, 0.61, 0.58 and 0.32 (Hibi et al., 1984) or as 14.28, with segments of 2.63, 2.12, 1.98, 1.61, 1.49, 0.99, 0.87, 0.80, 0.56, 0.50, 0.47 and 0.26 (Boccardo et al., 1984).
Protein: Seven proteins with estimated M. Wts (x 10-3) of 183, 165, 150, 143, 120, 56 and 45 (Omura et al., 1985). An RNA-dependent RNA polymerase is associated with the virus particles (Yokoyama et al., 1984).
Relations with Cells and Tissues
Virus particles are observed in the cytoplasm of phloem parenchyma cells of infected rice plants (Fig. 6). Virus particles were not found in the nucleus, chloroplasts or mitochondria.
Notes
Four viruses in the plant reovirus group infect rice: rice gall dwarf virus, rice dwarf virus (Iida et al., 1972), rice black-streaked dwarf virus (Shikata, 1974) and rice ragged stunt virus (Milne et al., 1982). Leafhoppers (Nephotettix spp.) transmit rice gall dwarf virus and rice dwarf virus in a similar manner (Inoue & Omura, 1982; Morinaka et al., 1982; Iida et al., 1972) and it is difficult to distinguish between these viruses by transmission tests.
Rice black-streaked dwarf virus and rice ragged stunt virus are transmitted by the planthoppers Laodelphax striatellus or Nilaparvata lugens respectively, and not by leafhoppers.
The four virus diseases can be identified by symptoms in the greenhouse at temperatures between 24 and 30°C. Rice dwarf virus is easily distinguishable from the rest because it does not produce galls (enations) but causes white or chlorotic specks on the foliage (Iida et al., 1972). Large numbers of small galls appear about 20 days after inoculation in rice infected with rice gall dwarf virus (Putta et al., 1980; Omura et al., 1980) whereas a few elongated galls appear from 50-60 days post inoculation in plants infected with rice black-streaked dwarf virus or rice ragged stunt virus (Shikata, 1974; Milne et al., 1982). The colour of the galls changes to dark brown at later stages of infection with rice black-streaked dwarf virus (Shikata, 1974) but no colour change occurs with rice gall dwarf or rice ragged stunt viruses.
No serological cross-reactivity has been reported for the four viruses. Latex flocculation tests (Omura et al., 1984) or enzyme-linked immunosorbent assay (Omura et al., 1984) is recommended for identification. The M. Wt distribution of the nucleic acid species is characteristic for each virus (Hibi et al., 1984; Boccardo et al., 1984).
Figures

Infected (left) and healthy (right) rice plants cv. Taichung Native 1 of the same age inoculated at 3-leaf stage.
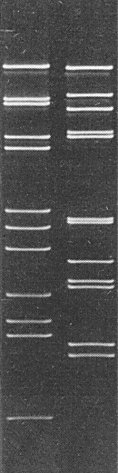
Polyacrylamide gel electrophoretic separation of the dsRNA segments of rice gall dwarf virus (left) and rice dwarf virus (right).
References list for DPV: Rice gall dwarf virus (296)
- Boccardo & Milne, CMI/AAB Descr. Pl. Viruses 294, 7 pp.,1984.
- Boccardo, Milne, Disthaporn, Chettanachit & Putta, Intervirology 23: 167, 1984.
- Faan, Chang, Ho, Xie, Liu, Zhou, Liu & Zhu, Guangdong Agric. Sci. 44: 41, 1983.
- Fenner & Gibbs, Intervirology 19:121, 1983.
- Hibi, Omura & Saito, J. gen. Virol. 65: 1585, 1984.
- Iida, Shinkai & Kimura, CMI/AAB Descr. Pl. Viruses 102, 4 pp.,1972.
- Inoue & Omura, Pl. Dis. 66: 57, 1982.
- Milne & Lovisolo, Adv. Virus Res. 21: 223, 1977.
- Milne, Boccardo & Ling, CMI/AAB Descr. Pl. Viruses 248, 5 pp.,1982.
- Morinaka, Putta, Chettanachit, Parajarearn, Disthaporn, Inoue & Omura, Pl. Dis., 66: 703, 1982.
- Omura, Inoue, Morinaka, Saito, Chettanachit, Putta, Parejarearn & Disthaporn, Pl. Dis. 64: 795, 1980.
- Omura, Kimura, Tsuchizaki & Saito, Ann. phytopath. Soc. Japan 48: 389, 1982a.
- Omura, Morinaka, Inoue & Saito, Phytopathology 72: 1246,1982b.
- Omura, Minobe, Kimura, Hibino, Tsuchizaki & Saito, Ann. phytopath. Soc. Japan 49: 670, 1983.
- Omura, Hibino, Usugi, Inoue, Morinaka, Tsurumachi, Ong, Putta, Tsuchizaki & Saito, Pl. Dis. 68: 374, 1984.
- Omura, Minobe, Matsuoka, Nozu, Tsuchizaki & Saito, J. gen. Virol. 66: 811, 1985.
- Ong & Omura, Int. Rice Res. Newsl. 7 (2): 7, 1982.
- Putta, Chettanachit, Morinaka, Parejarearn & Disthaporn, Int. Rice Res. Newsl. 5 (3): 10, 1980.
- Putta, Chettanachit, Omura, Inoue, Morinaka, Honda, Saito & Disthaporn, Int. Rice Res. Newsl. 7 (6): 13, 1982.
- Shikata, CMI/AAB Descr. Pl. Viruses 135, 4 pp., 1974.
- Yokoyama, Nozu, Hashimoto & Omura, J. gen. Virol. 65: 533, 1984.