Details of DPV and References
DPV NO: 306 September 1985
Family: Secoviridae
Genus: Nepovirus
Species: Cherry leaf roll virus | Acronym: CLRV
This is a revised version of DPV 80
Cherry leaf roll virus
A. T. Jones Scottish Crop Research Institute, Invergowrie, Dundee DD2 5DA, UK
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
The virus was described as elm mosaic by Swingle et al. (1941,1943) and Varney & Moore (1952) and as cherry leaf roll by Posnette & Cropley (1955) and Cropley (1961). Symptoms of walnut blackline disease, which is now also known to be caused by this virus (Mircetich & Rowhani, 1984), were first described by Schuster & Miller (1933) but not then attributed to an infective agent.
- Synonyms
- None. Many strain names used.
A virus with isometric particles c. 28 nm in diameter, common in many wild and cultivated woody plant species in Europe, the USSR, North America and New Zealand. It is readily transmitted by mechanical inoculation of sap and by seed to progeny of naturally and experimentally infected plants. It has a wide experimental host range and shows many features of nepoviruses but appears not to be transmitted by nematodes. There is evidence that at least some strains may be transmitted in pollen to the plant pollinated.
Main Diseases
Causes leaf patterns and blackline disease of walnut (Savino et al., 1977; Cooper, 1979, 1980; Mircetich et al., 1980; Mircetich & Rowhani, 1984; Fig. 1); chlorotic mosaic, ring patterns and dieback in American elm (Swingle et al., 1943; Mayhew & Epstein, 1971); leaf rolling and plant death in cherry (Cropley, 1961; Schimanski et al., 1975; Fig. 2); chlorotic ringspot, leaf patterns and/or yellow vein netting in Betula spp. (Schmelzer, 1972a; Cooper & Atkinson, 1975; Fig. 3), Sambucus spp. (Schmelzer, 1966; Schimanski & Schmelzer, 1972; Hansen & Stace-Smith, 1971), Rubus spp. (Cropley & Tomlinson, 1971; Jones, 1976; Jones & Wood, 1978) and Cornus florida (Waterworth & Lawson, 1973); and yellow spotting of Ptelea trifoliata (Schmelzer, 1972b). It is associated with ringspot symptoms in privet (Schmelzer, 1972b) and, when present with arabis mosaic virus, with chlorotic ringspot of lilac (Novak & Lanzova, 1975). Symptomless natural infection occurs in olive (Savino & Gallitelli, 1981), rhubarb (Tomlinson & Walkey, 1967), Berteroa incana (Lockhart, 1977), Delphinium elatum (Ahmed & Bailiss, 1975) and Rumex obtusifolius (Walkey & Cooper, 1973).
Geographical Distribution
Found in Europe and North America (Cropley & Tomlinson, 1971), Turkey (Kurcman, 1977), the USSR (Bubaker & Pomazkov, 1978) and New Zealand (Jones & Wood, 1978).
Host Range and Symptomatology
All isolates tested have wide experimental host ranges; hosts are reported in more than 36 plant families (Schmelzer, 1966; Hansen & Stace-Smith, 1971; Horvath, 1979), including the monocotyledons Tinantia erecta and Commelina spp. (Schmelzer, 1966; Horvath, 1979).
- Diagnostic species
- Chenopodium amaranticolor, C. quinoa. Chlorotic or necrotic local lesions
followed by systemic mottle or necrosis and distortion.
- Cucumis sativus (cucumber). Chlorotic local lesions in cotyledons followed occasionally by systemic mosaic.
- Nicotiana rustica, N. tabacum (tobacco) cvs White Burley, Xanthi-nc. Necrotic local lesions in 3-4 days, frequently developing into concentric necrotic rings (Fig. 5); systemic necrotic or chlorotic rings and line-patterns depending on the virus strain and growing conditions. The plants usually recover to produce symptomless new leaves.
- Cucumis sativus (cucumber). Chlorotic local lesions in cotyledons followed occasionally by systemic mosaic.
- Propagation species
- Nicotiana rustica or N. tabacum cvs White Burley and
Xanthi-nc are suitable for maintaining virus cultures. N.
clevelandii and Chenopodium quinoa are
the species most often used for propagating the virus for purification.
- Assay species
- Nicotiana tabacum cv. White Burley or Xanthi-nc and Chenopodium amaranticolor are good local lesion hosts.
Strains
Many strains have been reported. Isolates from different natural host species are serologically distinguishable from each other but most isolates from a single host species are not (Fig. 4; Jones & Murant, 1971; Jones, 1976). However, isolates of more than one serological type have been reported from privet (Schmelzer, 1972b), dogwood (Walkey et al., 1973), walnut (Savino et al., 1977; Cooper & Edwards, 1980) and American elm (B. Lockhart, personal communication). Isolates that cannot be differentiated serologically may differ in virulence both in natural and experimental hosts. The following are the strains that have been most studied:
- Type (cherry) strain (Cropley, 1961).
- Elm mosaic strain (Varney & Moore, 1952).
- Rhubarb strain (Tomlinson & Walkey, 1967).
- Golden elderberry strain (Hansen & Stace-Smith, 1971).
- Red elder ringspot strain (Schmelzer, 1972b).
- Dogwood ringspot strain (Waterworth & Lawson, 1973).
- Birch strain (Cooper & Atkinson, 1975).
- Walnut ringspot and walnut yellow vein strains (Savino et al., 1977).
- Blackberry and red raspberry strains (Cropley & Tomlinson, 1971; Jones & Wood, 1978).
- Elm mosaic strain (Varney & Moore, 1952).
Transmission by Vectors
Transmission of cherry isolates by the nematodes Xiphinema coxi, X. diversicaudatum and X. vuittenezi was reported (Fritzche & Kegler, 1964; Flegg, 1969). This has not been substantiated in more rigorous tests with the cherry, golden elderberry and rhubarb strains, nor were these strains transmitted by X. americanum, X. bakeri, Longidorus elongatus, L. leptocephalus, L. macrosoma or Paralongidorus maximus (van Hoof, 1971; Jones et al., 1981). The elm mosaic strain was not transmitted by X. americanum (Fulton & Fulton, 1970) or by the aphid Myzus persicae (Ford et al., 1972).
Transmission through Seed
Seed-borne (0.5-35%) in most natural hosts tested, including American elm (Callahan, 1957a, 1957b), Betula spp. (Cooper, 1976; Schimanski et al., 1980), Prunus spp. (Schimanski et al., 1975, 1976), rhubarb (Tomlinson & Walkey, 1967), Sambucus spp. (Schimanski & Schmelzer, 1972) and walnut (Quacquarelli & Savino, 1977; Cooper & Edwards, 1980). The elm mosaic and birch strains are transmitted both through pollen and through the ovule to the seed of their natural hosts (Callahan, 1957a, 1957b; Cooper et al., 1984). There is some evidence in birch that infected pollen introduced the virus into the pollinated tree (Cooper et al., 1984). Strong circumstantial evidence indicates that spread of the walnut strain in mature Californian walnut orchards is through pollen (Mircetich et al., 1980); up to 14% of healthy walnut trees hand-pollinated with infected pollen became infected (S. M. Mircetich & A. Rowhani, unpublished data). Seed-borne up to 100% in many herbaceous hosts (Schmelzer, 1965; Lister & Murant, 1967; Hansen & Stace-Smith, 1971; Cooper, 1976; Murant, 1983).
Transmission by Dodder
An elderberry strain was not transmitted by Cuscuta californica, C. campestris or C. subinclusa (Schmelzer, 1966).
Serology
The virus is moderately immunogenic giving rabbit antiserum with titres up to 1/2000. In gel double diffusion tests the virus produces one band of precipitate and in spring it can be detected by this means in cherry sap (Cropley, 1960). Enzyme-linked immunosorbent assay and immunosorbent electron microscopy have been used to detect infection in tissues of natural and experimental hosts (Jones & Duncan, 1980; Cooper et al., 1984; Massalski & Cooper, 1984; Rowhani et al., 1985).
Relationships
The properties of its particles place cherry leaf roll virus in the nepovirus group but it appears not to be transmitted by nematodes (Jones et al., 1981). Varney & Moore (1952) found that the elm mosaic strain failed to induce lesions in plants previously infected with tomato ringspot virus. Jones (1976) confirmed this observation with eight strains of cherry leaf roll virus in Nicotiana tabacum cv. Xanthi-nc but found that none of the strains protected plants against lesion production by tomato ringspot virus. Although the results of their plant protection tests led Varney & Moore (1952) to suspect a relationship with tomato ringspot virus, serological tests have shown the viruses to be distinct (Fulton & Fulton, 1970; Jones & Murant, 1971; Jones & Duncan, 1980). Furthermore, no serological relationship was detected to the nepoviruses grapevine Bulgarian latent, lucerne Australian latent or peach rosette mosaic viruses, which all have RNA-2 species of M. Wt similar to that of cherry leaf roll virus (Jones & Duncan, 1980), nor to arabis mosaic, grapevine fan leaf, raspberry ringspot, strawberry latent ringspot, tobacco ringspot, tomato black ring, tomato ringspot or tomato tip necrosis viruses (Cropley, 1961; Hansen & Stace-Smith, 1971; Jones & Murant, 1971).
Stability in Sap
In sap of infected Nicotiana clevelandii or N. tabacum, infectivity is lost after 10 min at 50-60°C, dilution to 10-3-10-5 or storage at 20°C for 4-16 days (Cropley, 1961; Walkey et al., 1973; Jones, 1976). Strains from elm and dogwood seem less stable than most other strains (Jones, 1976). In elm pollen, the elm mosaic strain was infective after 3 years at 0°C (Callahan, 1957b).
Purification
Some strains are more difficult to purify than others. The following method was successful in purifying several strains (Jones, 1973; Cooper & Atkinson, 1975).
Extract infected Chenopodium quinoa or Nicotiana clevelandii
leaves in 0.5 M borate buffer, pH 6.5 (1 g leaf: 2 ml buffer), freeze the slurry
overnight, thaw and centrifuge at 10,000 g for 10 min. Add 15 g
granulated ammonium sulphate to each 100 ml supernatant fluid and stir overnight
at 4°C. Centrifuge at 10,000 g for 10 min and concentrate the virus
from the supernatant fluid by differential centrifugation and sucrose density
gradient centrifugation.
An alternative method (Walkey et al., 1973) is to extract leaves in 0.5
M sodium citrate buffer, pH 6.5, and
chloroform (1 g:1 ml:1 ml). Add polyethylene glycol (M. Wt 6000) to the aqueous
phase to 6% (w/v) and stir for 2 h
before centrifuging at 10,000 g for 10 min. Resuspend the
virus-containing pellet in 0.01 M Tris-HCl, pH 7.3.
Concentrate the virus by differential centrifugation and sucrose density gradient centrifugation.
Properties of Particles
Particles in purified preparations sediment as two nucleoprotein components (M, B). Preparations of some strains also contain an RNA-free component (T). The ratio of M:B component differs with the virus strain (Jones, 1973; Haber & Hamilton, 1980).
Sedimentation co-efficients (s20,w, svedbergs): 52 (T), 115 (M), 128 (B).
Particle weights (daltons x 10-6): 3.24 (T), 5.53 (M), 6.06 (B).
Diffusion coefficient (D20,w): calculated to be 1.48 x 10-7 cm2/s (Murant et al., 1981).
Electrophoretic mobility: at pH 6.5 the elm mosaic and dogwood ringspot strains migrated to the anode whereas the cherry, golden elderberry and rhubarb strains migrated to the cathode. All five strains appeared to contain two electrophoretic components; these did not correspond to the M and B sedimenting components (Walkey et al., 1973).
A260/A280: 1.62 (mixture of M and B components).
Buoyant density in CsCl (g/cm3): 1.47 (M), 1.50 (B).
Particle Structure
Isometric, c. 28 nm in diameter, angular in outline (Fig. 7). A proportion of the particles is penetrated by negative stain (Fig. 7), and these presumably correspond to the T component.
Particle Composition
Nucleic acid: RNA, single-stranded; two genomic species, RNA-1 and RNA-2, packaged separately in B particles and M particles respectively, and comprising 46% (B) and 41% (M) of the particle weight. In polyacrylamide gels under non-denaturing conditions their estimated M. Wt was 2.4 x 106 (RNA-1) and 2.1 x 106 (RNA-2) (Jones & Mayo, 1972; Walkey et al., 1973). In agarose gels the estimated M. Wts of glyoxal-denatured RNA molecules were 2.82 x 106 and 2.29 x 106 respectively (Murant et al., 1981). In the golden elderberry strain, the molar percentage of nucleotides for RNA-1 was G26.5; A22.0; C22.3; U29.1. For RNA-2 it was G26.6; A21.4; C22.8; U29.2 (Walkey et al., 1973).
Protein: In polyacrylamide/SDS gels, protein preparations from all strains studied contain a single polypeptide species of estimated M. Wt 54,000 (Jones & Mayo, 1972; Walkey et al.,1973). The particles are assumed to contain 60 such subunits which comprise 59% and 54% of the weights of M and B particles respectively (Jones & Mayo, 1972; Murant et al., 1981). The amino-acid compositions of the particle proteins of five strains have been determined (Walkey et al., 1973).
Genome Properties
Both RNA species are required for infectivity (Jones & Mayo, 1972; Jones & Duncan, 1980; Haber & Hamilton, 1980). They both contain polyadenylate sequences (Massalski, 1984) and a genome-linked protein of estimated M. Wt 3500 (C. U. T. Hellen & J. I. Cooper, personal communication). In pseudo-recombinant isolates made between the golden elderberry and rhubarb strains, RNA-2 determined serological specificity, the ratio of M : B components and the production of T component; RNA-1 determined the ability to infect Gomphrena globosa (Jones & Duncan, 1980; Haber & Hamilton, 1980). Haber & Hamilton (1980) found that RNA-2 determined the type and severity of symptoms in species of Chenopodium and Nicotiana whereas Jones & Duncan (1980) found that RNA-1 was the major determinant of these characters but that RNA-2 had a modifying influence. Determinants on both RNA-1 and RNA-2 were involved in plant protection (Jones & Duncan, 1980).
Relations with Cells and Tissues
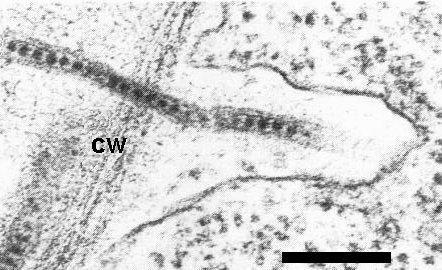
In Nicotiana spp. the virus was detected within tubules in pollen, ovules, mature seeds, root and shoot meristems and leaf cells (Fig. 6; Walkey & Webb, 1968, 1970; Jones et al., 1973). Leaf cells also contained inclusion bodies consisting of membraneous structures, endoplasmic reticulum and ribosomes (Jones et al., 1973). In naturally infected plants, virus-like particles within tubules were detected in leaf cells (Cooper & Atkinson, 1975), in the unfertilized female gametophyte and embryo of birch (Cooper et al., 1984), and in pollen grains of birch (Massalski & Cooper, 1984). Virus was also detected by ELISA on the surface of pollen grains from infected birch, cherry and walnut (Massalski & Cooper, 1984). Virus-like particles were never detected in the nucleus of infected cells. Infected cells often possessed cell wall projections (Jones et al., 1973; Cooper & Atkinson, 1975); in symptom-bearing leaves of birch, chloroplasts often contained large densely staining plastoglobuli and had poorly defined lamellae (Cooper & Atkinson, 1975).
The virus can be eliminated from plants by meristem tip culture (Walkey et
al., 1969) and by maintaining seedlings for 7 days at 40°C or 20 days at 32°C
(Cooper & Walkey, 1978).
Notes
In natural and experimental host plants the virus induces symptoms resembling those produced by many nepoviruses and can therefore be identified unequivocally only by serological tests. Because of the wide range of serological variants, antisera to several virus strains should be used for identification.
Figures

Blackline symptom at the graft union of infected English walnut grafted on Juglans hindsii rootstock (figure courtesy of S. M. Mircetich).

Stunting and leaf roll symptoms in two Prunus avium cv. Early Rivers (sweet cherry) trees (right). Uninfected plants on the left.(Figure courtesy of East MaIling Research Station.)

Systemic chlorotic mottle, ringspots and line patterns in leaves of Betula pendula following mechanical inoculation (figure courtesy of J. 1. Cooper).

Agarose gel double diffusion plate. Centre well contains antiserum to the golden elderberry strain of cherry leaf roll virus, peripheral wells contain sap of Chenopodium quinoa infected with isolates from golden elderberry (6), American elder(S), rhubarb (R), cherry (C) and elm (E).

Local necrotic lesions with concentric necrotic rings in Nicotiana tabacum cv. White Burley (figure courtesy of National Vegetable Research Station).
References list for DPV: Cherry leaf roll virus (306)
- Ahmed & Bailiss, J. hort. Sci. 50: 47, 1975.
- Bubaker & Pomazkov, Izv. timiryazev. sel'-khoz. Akad. 5: 219, 1978.
- Callahan, Diss. Abstr. 17: 1861, 1957a.
- Callahan, Phytopathology 47: 5, 1957b.
- Cooper, Mitt. biol. BundAnst. Ld- u. Forstw. 170: 17, 1976.
- Cooper, Virus Diseases of Trees and Shrubs, Institute of Terrestrial Ecology, Cambridge. 74 pp., 1979.
- Cooper, Acta phytopath. Acad. Sci. hung. 15: 139, 1980.
- Cooper & Atkinson, Forestry 48: 193, 1975.
- Cooper & Edwards, Forestry 53: 41, 1980.
- Cooper & Walkey, Ann. appl. Biol. 88: 273, 1978.
- Cooper, Massalski & Edwards, Ann. appl. Biol. 105: 55, 1984.
- Cropley, Nature, Lond. 188: 875, 1960.
- Cropley, Ann. appl. Biol. 49: 524, 1961.
- Cropley & Tomlinson, CMI/AAB Descr. Pl. Viruses 80, 4 pp., 1971.
- Flegg, Rep. E. Malling Res. Stn for 1968: 155, 1969.
- Ford, Moline, McDaniel, Mayhew & Epstein, Phytopathology 62: 987, 1972.
- Fritzsche & Kegler, Naturwissenschaften 51: 299, 1964.
- Fulton & Fulton, Phytopatholagy 60: 114, 1970.
- Haber & Hamilton, J. gen. Virol. 50: 377, 1980.
- Hansen & Stace-Smith, Phytopathology 61: 1222, 1971.
- Horváth, Acta phytopath. Acad. Sci. hung. 14: 319, 1979.
- Jones, Ann. appl. Biol. 74: 211, 1973.
- Jones, Paljopr. Znan. Smotra 39: 527, 1976.
- Jones & Duncan, J. gen. Virol. 50: 269, 1980.
- Jones & Mayo, J. gen. Virol. 16: 349, 1972.
- Jones & Murant, Ann. appl. Biol. 69: 11, 1971.
- Jones & Wood, Pl. Dis. Reptr 62: 835, 1978.
- Jones, Kinninmonth & Roberts, J. gen. Virol. 18: 61, 1973.
- Jones, McElroy & Brown, Ann. appl. Biol. 99: 143, 1981.
- Kurcman, Bitki Koruma Bült. 17: 113, 1977.
- Lister & Murant, Ann. appl. Biol. 59: 49, 1967.
- Lockhart. Phytopathology 4: 90, 1977.
- Massalski, Ph.D Thesis, Univ. Oxford, 246 pp., 1984.
- Massalski & Cooper, Pl. Path. 33: 255, 1984.
- Mayhew & Epstein, Phytopathology 61: 1024, 1971.
- Mircetich & Rowhani, Phytopathology 74: 423, 1984.
- Mircetich, Sanborn & Ramos, Phytopathology 70: 962, 1980.
- Murant, Seed Sci. Technol. 11: 973, 1983.
- Murant, Taylor, Duncan & Raschké J. gen. Virol. 53: 321, 1981.
- Novak & Lanzova, Biol. Plantarum 17: 226, 1975.
- Posnette & Cropley, Rep. E. Malling Res. Stn for 1954: 126, 1955.
- Quacquarelli & Savino, Phytopath. Mediterranea 16: 154, 1977.
- Rowhani, Mircetich, Shepherd & Cucuzza, Phytopathology 75: 48, 1985.
- Savino & Gallitelli, Phytopath. Mediterranea 20: 202, 1981.
- Savino, Quacquarelli, Gallitelli, Piazolla & Martelli, Phytopath. Mediterranea 16: 96, 1977.
- Schimanski & Schmelzer, Zentbl. Bakt. ParasitKde. Abt. 2 127: 673, 1972.
- Schimanski, Schmelzer & Albrecht, Arch. PflSchutz 5: 329, 1975.
- Schimanski, Schmelzer & Albrecht, Zent. Bakt. ParasitKde, Abt. 2 131: 117, 1976.
- Schimanski, Albrecht & Kegler, Arch. PflSchutz 3: 231, 1980.
- Schmelzer, Zast. Bilja 85-88: 485, 1965.
- Schmelzer, Phytopath. Z. 55: 317, 1966.
- Schmelzer, Zent. Bakt. ParasitKde, Abt. 2 127: 10, 1972a.
- Schmelzer, Zent. Bakt. ParasitKde, Abt. 2 127: 140, 1972b.
- Schuster & Miller, Phytopathology 23: 408, 1933.
- Swingle, Tilford & Irish, Phytopathology 31: 22, 1941.
- Swingle, Tilford & Irish, Phytopathology 33: 1196, 1943.
- Tomlinson & Walkey, Ann. appl. Biol. 59: 415, 1967.
- Van Hoof, Neth. J. Pl. Path. 77: 30, 1971.
- Varney & Moore, Phytopathology 42: 476, 1952.
- Walkey & Cooper, Rep. natn. Veg. Res. Stn for 1972: 100, 1973.
- Walkey & Webb, J. gen. Virol. 3: 311, 1968.
- Walkey & Webb, J. gen. Virol. 7:159, 1970.
- Walkey, Fitzpatrick & Woolfit, J. gen. Virol. 5: 237, 1969.
- Walkey, Stace-Smith & Tremaine, Phytopathology 63: 566, 1973.
- Waterworth & Lawson, Phytopathology 63: 141, 1973.