Details of DPV and References
DPV NO: 309 September 1985
Family: Secoviridae
Genus: Nepovirus
Species: Tobacco ringspot virus | Acronym: TRSV
This is a revised version of DPV 17
Tobacco ringspot virus
R. Stace-Smith Agriculture Canada Research Station, 6660 N.W. Marine Drive, Vancouver, B.C., Canada V6T 1X2
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
Described by Fromme et al. (1927)
- Selected synonyms
- Tobacco ringspot virus no. 1 (Rev. appl. Mycol. 15:
831)
- Annulus tabaci (Rev. appl. Mycol. 28: 514)
- Nicotiana virus 12 (Rev. appl. Mycol. 36: 303)
- Annulus tabaci (Rev. appl. Mycol. 28: 514)
- A virus with isometric particles about 28 nm in diameter sedimenting
as three components and with a bipartite RNA genome. The virus is readily
transmissible by sap inoculation and has a wide host range, including both
woody
and herbaceous plants. It is transmitted by the nematode Xiphinema
americanum and other closely related Xiphinema spp. Reports of
natural spread are largely confined to North America but the virus has been
disseminated to many countries in infected planting material. A satellite
RNA is associated with some virus cultures.
Main Diseases
The virus occurs in nature in both annual and perennial crops. It causes serious disease problems in those regions of North America where the nematode vectors also occur. If the virus is disseminated in seed or planting stock to areas where Xiphinema spp. are absent, or present in low numbers, the disease problem is usually negligible. Of the many diseases caused by the virus, bud blight of soybean is the most severe and causes the greatest losses. The most obvious symptom is curving of the terminal bud, with other buds on the infected plant later becoming brown and brittle. Brown streaks may develop on the stems and petioles of larger leaves, pods are underdeveloped and aborted, and those that set before infection may develop dark blotches (Sinclair & Shurtleff, 1975). The virus is widespread in the tobacco-growing areas of North America, causing ring and line patterns on the foliage, dwarfing of the plant, and small leaves of poor quality (Lucas, 1975). The virus occurs in the major blueberry-growing region of the USA, causing blueberry necrotic ringspot disease, in which susceptible cultivars are stunted, unproductive, show extensive twig dieback, and necrotic or chlorotic spots, rings or line patterns on the foliage (Converse & Ramsdell, 1982; Lister et al., 1963). The virus causes a ringspot disease of cucurbits in Texas (McLean & Meyer, 1961) and Wisconsin (Sinclair & Walker, 1956). Infected plants are stunted and show a leaf mottle accompanied by leaf malformation and reduced fruit set. Other plants that have been found naturally infected with the virus include American spearmint (Stone et al., 1962), blackberry (Rush & Gooding, 1970), cherry (Fig. 1; Stace-Smith & Hansen, 1974), apple (Lana et al., 1983), grapevine (Gilmer et al., 1970), pelargonium (Kemp, 1967), ash (Hibben & Bozarth, 1972), dogwood (Waterworth & Povish, 1972), anemone (Hollings, 1965), gladiolus (Bridgmon & Walker, 1952) and elderberry (Wilkinson, 1952).
Geographical Distribution
The virus is endemic in central and eastern North America, extending from southern Ontario in Canada to the Rio Grande Valley in Texas. Although the virus has been isolated from cherry (Stace-Smith & Hansen, 1974) and blueberry (Converse & Ramsdell, 1982) in western North America, the apparent absence there of field spread suggests that infected plants were introduced as nursery stock. Similarly, the virus has been isolated from various plant species in other parts of the world, including gladiolus in Japan (Fukumoto et al., 1982) and Australia (Randles & Francki, 1965), iris in the Netherlands (Asjes, 1979), petunia (Rani et al., 1969), capsicum (Bidari & Reddy, 1983), eggplant (Sastry & Nayudu, 1976) and soybean (Gupta, 1978) in India, papaya in Nigeria (Lana, 1980), soybean (Murav'eva, 1976) and lupin (Kowalska, 1971) in the USSR, tobacco in Yugoslavia (Mickovski, 1969) and cucurbits in Iran (Ebrahim-Nesbat, 1974). As the natural vectors of the virus occur in various parts of the world, limited natural spread may be associated with some of the recorded occurrences outside the USA and Canada.
Host Range and Symptomatology
Experimental host range is wide; species in more than 17 dicotyledonous and monocotyledonous families are susceptible (Price, 1940). In nature, the virus occurs both in woody and in herbaceous plants. The virus is transmitted by inoculation with sap, readily to herbaceous hosts but with difficulty to woody hosts.
- Diagnostic species
- Chenopodium amaranticolor and C. quinoa. Necrotic local
lesions; usually no systemic infection.
Cucumis sativus (cucumber). Chlorotic or necrotic local lesions; systemic mottling and dwarfing with apical distortion (Fig. 2).
- Nicotiana tabacum (tobacco) (Fig. 3) and N. clevelandii (Fig. 4). Necrotic local lesions that frequently develop into rings or ringspots; systemically infected leaves may show ring or line patterns. Leaves produced later are symptomless but contain virus.
- Phaseolus vulgaris (French bean). Necrotic spots on inoculated leaves; systemically infected leaves may show spots and rings and the growing tip becomes necrotic.
- Lycopersicon esculentum (tomato). Difficult to infect by sap inoculation; plants that are infected develop small necrotic spots on inoculated leaves, systemic vein necrosis and epinasty.
- Vigna unguiculata ssp. sinensis (cowpea). Necrotic primary lesions, systemic necrosis, apical necrosis and wilt. Cowpea cultivars may be used to distinguish strains. Local lesions may be greatly decreased in size or show other modifications when satellite RNA is present in the inoculum (Schneider, 1971; Schneider et al., 1972b).
- Propagation species
- Nicotiana spp. are suitable for maintaining cultures; N.
clevelandii or cucumber are good sources of virus for purification.
- Assay species
- Nicotiana tabacum, N. clevelandii, Chenopodium amaranticolor and Vigna unguiculata are useful for local lesion assays. Cucumber is a useful source and bait plant for nematode transmission experiments (Fulton, 1962).
- Asjes, Neth. J. Pl. Path. 85: 269, 1979.
- Athow & Bancroft, Phytopathology 49: 697, 1959.
- Bergeson, Athow, Laviolette & Thomasine, Phytopathology 54: 723, 1964.
- Bidari & Reddy, Pl. Path. Newsl. 1: 11, 1983.
- Bridgmon & Walker, Phytopathology 42: 65, 1952.
- Chu & Francki, Virology 93: 398, 1979.
- Chu, Boccardo & Francki, Virology 109: 428, 1981.
- Converse & Ramsdell, Pl. Dis. 66: 710, 1982.
- Crowley, Davison, Francki & Owusu, Virology 39: 322, 1969.
- Davison & Francki, Virology 39: 235, 1969.
- Douthit & McGuire, Pl. Dis. Reptr 62: 164, 1978.
- Dunleavy, Phytopathology 47: 681, 1957.
- Ebrahim-Nesbat, Phytopath. Z. 79: 352, 1974.
- Forster & Morris-Krsinich, Virology 144: 516, 1985.
- Fribourg, Phytopathology 67: 174, 1977.
- Fromme, Wingard & Priode, Phytopathology 17: 321, 1927.
- Fukumoto, Ito & Tochihara, Ann. phytopath. Soc. Japan 48: 68, 1982.
- Fulton, Phytopathology 52: 375, 1962.
- Gergerich, Asher & Ramsdell, Phytopath. Z. 107: 289,1983.
- Gilmer, Uyemoto & Kelts, Phytopathology 60: 619, 1970.
- Gooding, Phytopathology 60: 708, 1970.
- Gupta, Acta bot. ind. 6: 169, 1978.
- Halk & McGuire, Phytopathology 63: 1291, 1973.
- Harrison & Barker, J. gen. Virol. 40: 711, 1978.
- Harrison & Murant, CMI/AAB Descr. Pl. Viruses 185, 4 pp., 1977.
- Harrison, Murant & Mayo, J. gen. Virol. 17: 137, 1972.
- Hibben & Bozarth, Phytopathology 62: 1023, 1972.
- Hollings, Ann. appl. Biol. 55: 447, 1965.
- Hollings & Stone, Ann. appl. Biol. 65: 411, 1970.
- Kahn, Scott & Monroe, Phytopathology 52: 1211, 1962.
- Kemp, Can. J. Pl. Sci. 47: 295, 1967.
- Kiefer, Daubert, Schneider & Bruening, Virology 121: 262, 1982.
- Komuro & Iwaki, Ann. phytopath. Soc. Japan 34: 7, 1968.
- Kowalska, Roczn. Nauk roln., Ser. E 1:17, 1971.
- Ladipo & de Zoeten, Phytopathology 62: 195, 1972.
- Lamberti & Bleve-Zacheo, Nematol. Mediterranea 7: 51, 1979.
- Lana, J. hort. Sci. 55: 191, 1980.
- Lana, Peterson, Rouselle & Vrain, Phytopath. Z. 106: 141, 1983.
- Linthorst & Kaper, Virology 137: 216, 1984.
- Lister, Phytopathology 68: 1393, 1978.
- Lister, Raniere & Varney, Phytopathology 53: 1031, 1963.
- Lucas, Diseases of Tobacco, Raleigh: Biological Consulting Associates, 621 pp., 1975.
- Mandahar, in Plant Diseases and Vectors: Ecology and Epidemiology, p. 241, eds K. Maramorosch and K. F. Harris, New York: Academic Press, 368 pp., 1981.
- Mayo, Murant & Harrison, J. gen Virol. 12: 175, 1971.
- Mayo, Barker & Harrison, J. gen Virol. 43: 603, 1979a.
- Mayo, Barker & Harrison, J. gen Virol. 43: 735, 1979b.
- Mayo, Barker & Harrison, J. gen Virol. 59: 149, 1982.
- McGuire, Phytopathology 54: 799, 1964.
- McGuire & Douthit, Phytopathology 68: 457, 1978.
- McGuire, Kim & Douthit, Virology 42: 212, 1970.
- McKinney, Silber & Greeley, Phytopathology 55: 1043, 1965.
- McLean, Pl. Dis. Reptr 46: 877, 1962.
- McLean & Meyer, Pl. Dis. Reptr 45: 137, 1961.
- Messieha, Phytopathology 59: 943, 1969.
- Mickovski, Zast. Bilja 20: 203, 1969.
- Moore, Lister, Abney & Athow, Pl. Dis.66: 790, 1982.
- Murant, Seed Sci. Technol. 11: 973, 1983.
- Murant, Taylor, Duncan & Raschké, J. gen. Virol. 53: 321, 1981.
- Murav'eva, Zashch. Rast., Mosk. 11: 45, 1976.
- Newhart, Romaine & Craig, J. Am. Soc. hort. Sci. 107: 930, 1982.
- Owusu, Crowley & Francki, Ann. appl. Biol. 61: 195, 1968.
- Price, Phytopathology 26: 665, 1936.
- Price, Am. J. Bot. 27: 530, 1940.
- Ramsdell, Pl. Dis. Reptr 62: 1047, 1978.
- Randles & Francki, Aust. J. biol. Sci. 18: 979, 1965.
- Rani, Verma & Verma, Pl. Dis. Reptr 53: 903, 1969.
- Rezaian, Virology 100: 400, 1980.
- Roberts, Christie & Archer, Virology 42: 217, 1970.
- Romaine, Newhart & Anzola, Phytopathology 71: 308, 1981.
- Rush & Gooding, Phytopathology 60: 1756, 1970.
- Salazar & Harrison, Nature, Lond. 265: 337, 1977.
- Salazar & Harrison, Ann. appl. Biol. 90: 387, 1978.
- Sastry & Nayudu, Phytopath. Mediterranea 15: 60, 1976.
- Sauer, Phytopathology 56: 862, 1966.
- Schneider, Virology 45: 108, 1971.
- Schneider & Diener, Virology 29: 92, 1966.
- Schneider & Thompson, Virology 78: 453, 1977.
- Schneider, Hull & Markham, Virology 47: 320, 1972a.
- Schneider, White & Gooding, Virology 50: 902, 1972b.
- Schuster, Pl. Dis. Reptr 47: 510, 1963.
- Semal, Nature, Lond. 182: 1688, 1958.
- Sinclair & Shurtleff, Compendium of Soybean Diseases, St. Paul: American Phytopathological Society, 69 pp., 1975.
- Sinclair & Walker, Pl. Dis. Reptr 40: 19, 1956.
- Sogo & Schneider, Virology 117: 401, 1982.
- Sogo, Schneider & Koller, Virology 57: 459, 1974.
- Stace-Smith & Hansen, Can. J. Bot. 52: 1647, 1974.
- Stace-Smith, Reichmann & Wright, Virology 25: 487, 1965.
- Stanley, J. biol. Chem. 129: 405, 1939.
- Steere, Phytopathology 46: 60, 1956.
- Stone, Mink & Bergeson, Pl. Dis. Reptr 46: 623, 1962.
- Thomas, Phytopathology 59: 633, 1969.
- Tomlinson, Shepherd & Walker, Phytopathology 49: 293, 1959.
- Trudgill, Brown & McNamara, Revue Nématol. 6: 133, 1983.
- Tu, Phytopath. Z. 101: 153, 1981.
- Valleau, Bull. Ky agric. Exp. Stn 327: 43, 1932.
- Van Hoof, Neth. J. Pl. Path. 77: 30, 1971.
- Walkey & Webb, J. gen. Virol. 7: 159, 1970.
- Waterworth & Povish, Pl. Dis. Reptr 56: 336, 1972.
- Wilkinson, Phytopathology 42: 478, 1952.
- Yang & Hamilton, Virology 62: 26, 1974.
Strains
Two viruses originating in Peru, eucharis mottle (Kahn et al., 1962) and potato black ringspot (Salazar & Harrison, 1977) (= Andean potato calico virus of Fribourg (1977)) are serologically related to tobacco ringspot virus. However, the relationship is sufficiently distant for these viruses to be considered separate entities (Gooding, 1970; Salazar & Harrison, 1978). Many variants of tobacco ringspot virus have been reported, based primarily on differences in symptomatology (Valleau, 1932; Hollings, 1965; Sauer, 1966; Tu, 1981). There are also many natural antigenic variants: Gooding (1970) identified four serologically distinct isolates from tobacco and one from watermelon. No correlation was found between a variant and its geographical origin or the type of tobacco from which it was isolated.
Transmission by Vectors
Most vector studies have been done with nematode populations identified as Xiphinema americanum. However, X. americanum sensu lato is now considered to be a complex of many species (Lamberti & Bleve-Zacheo, 1979) and clarification of the vector potential of the component species is required. X. coxi has been reported to be a vector (van Hoof, 1971) but the evidence supporting this claim is considered to be inadequate (Trudgill et al., 1983).
The virus is acquired by X. americanum within 24 h. It is transmitted by adult and larval stages. Single nematodes may infect a plant but the frequency of infection increases with the number of nematodes (McGuire, 1964). Viruliferous nematodes could still transmit after storage at 10°C for 49 weeks (Bergeson et al., 1964). The nematodes transmitted the virus to 25 of 38 species tested, with 100% transmission occurring when the nematodes were given 3 weeks’ access to the infected source and 10 viruliferous nematodes were placed on each test plant (Douthit & McGuire, 1978). Virus-like particles were found in the lumen of the oesophagus of viruliferous nematodes (McGuire et al., 1970).
Transmission by X. americanum to soybean is inefficient (Bergeson et al., 1964; McGuire & Douthit, 1978), suggesting that other vectors may be involved. A possible important vector is Thrips tabaci, of which nymphs but not adults are capable of acquiring and transmitting the virus (Messieha, 1969). Other possible vectors are spider mites of the genus Tetranychus (Thomas, 1969), grasshoppers of the genus Melanoplus (Dunleavy, 1957) and the tobacco flea beetle, Epitrix hirtipennis (Schuster, 1963). There are also reports of aphid species serving as vectors (Semal, 1958; Komuro & Iwaki, 1968; Rani et al., 1969).
Transmission through Seed
Seed-transmission has been reported in at least 12 species of crop and weed hosts, the frequency of transmission ranging from 3% in Cucumis melo to 100% in Glycine max (Mandahar, 1981; Murant, 1983). Probably some seed-transmission occurs in most hosts. The virus is associated with the embryonic tissue of the seed but not with the seed coat. The age of the plant at the time of infection is the most important factor in determining the extent of seed-transmission (Athow & Bancroft, 1959; Owusu et al., 1968). Cross-pollination experiments with soybean suggest that infection of megagametophytes is the principal factor contributing to seed-transmission (Yang & Hamilton, 1974).
Serology
The virus is a good immunogen. Rabbits given a series of intravenous or intramuscular injections yield antiserum samples with titres up to 1/2048. A single precipitin band is formed in gel-diffusion tests. Serologically distinguishable strains have been identified in gel-diffusion tests (Gooding, 1970; Gergerich et al., 1983). The ELISA technique is applicable for detecting the virus in soybean (Lister, 1978; Moore et al., 1982) and pelargonium (Romaine et al., 1981; Newhart et al., 1982).
Relationships
The virus is the type member of the nepovirus group. Based on the occurrence of the smaller nucleic acid species in some particles of the bottom component as well as in those of the middle component, the virus is in a cluster of nepoviruses that contains arabis mosaic, eucharis mottle, grapevine fanleaf, potato black ringspot (= Andean potato calico virus) and raspberry ringspot viruses (Harrison & Murant, 1977). The virus is not related serologically to arabis mosaic, grapevine fanleaf or raspberry ringspot viruses but it is related to eucharis mottle and potato black ringspot viruses. In fact, these two viruses, both originating in Peru, have been considered as strains of tobacco ringspot virus (Kahn et al., 1962; Fribourg, 1977). However, the serological relationships are not close and this criterion together with behaviour in plant-protection tests and inability to form pseudo-recombinants suggests that they should be considered separate viruses (Salazar & Harrison, 1978).
In plant-protection tests, plants infected with one isolate usually do not develop additional symptoms after challenge inoculation with a second isolate (Price, 1936). An exception to this was obtained in plant-protection tests involving an isolate from ‘Jersey’ highbush blueberry, where tobacco plants initially inoculated with a tobacco isolate or a different blueberry isolate did not protect against the Jersey isolate when challenge-inoculated (Ramsdell, 1978).
Stability in Sap
In petunia, tobacco or French bean sap the virus loses infectivity after 10 min at 60-65°C, storage at room temperature for 1-2 weeks, or dilution to 10-3 to 10-4. Lyophilized sap was infective after storage for more than 10 years in sealed ampoules (Hollings & Stone, 1970) or more than 17 years in leaf tissue dehydrated over calcium chloride and stored at c. 1°C (McKinney et al., 1965).
Purification
The virus particles are relatively stable and many purification procedures are satisfactory. Squash, cucumber, petunia, cowpea, French bean, tobacco and N. clevelandii have been used as sources for purification (Steere, 1956; Stace-Smith et al., 1965; Ladipo & de Zoeten, 1972; Murant et al., 1981). Virus yield depends on the isolate, propagation host, time of year and purification procedure, but yields of 50-100 mg/kg tissue are not uncommon.
A purification procedure devised by Steere (1956) involved the addition of butanol and chloroform to the extracted sap at the rate of 1:1:1. This procedure has the disadvantage of using large quantities of organic solvents. A modification that is equally satisfactory involves the addition of n-butanol to a final concentration of 8.5% (Tomlinson et al., 1959). After clarification of leaf extracts by either of these methods, the virus may be concentrated by differential centrifugation.
Properties of Particles
The particles are all the same size but sediment as three components in sucrose density gradients (Fig. 5). The top component (T) consists of empty protein shells; the middle (M) and bottom (B) components are nucleoproteins containing different amounts of RNA. The B component particles are of two types containing either 1 molecule of RNA-1 or 2 molecules of RNA-2. Isolates containing the satellite RNA produce 11 to 14 additional types of particle containing different numbers of satellite RNA molecules.
Sedimentation coefficients (s20,w) in svedbergs: 53 (T), 91(M) and 126 (B) (Schneider & Diener, 1966; Fig. 6). Sedimentation coefficients of particles containing the satellite RNA range from 91 to 126 S (Schneider et al., 1972a).
Particle weight (daltons; assuming 60 subunits of M. Wt 57,000): 3.4 x 106 (T), 4.7 x 106 (M), 6.1 x 106 (B) (Mayo et al., 1971).
Diffusion coefficient (D20 x 10-7 cm2 sec-1): 1.59 (Stace-Smith et al., 1965).
Isoelectric point: pH 4.7 (Stanley, 1939).
Electrophoretic mobility: 11.3 x 10-5 cm2 volt-1 sec-1 in 0.05 M phosphate, pH 7.0 (Steere, 1956).
Absorption coefficient (A0.1%,1 cm) at 260 nm: about 10.0 (B) (R. Stace-Smith, unpublished data).
A260/A280: 0.72 (T), 1.38 (M), 1.57 (B) (R. Stace-Smith, unpublished data).
Buoyant density (g/cm3): 1.423 (M); bottom component has two populations averaging 1.507 (B1) and 1.518 (B2) (Schneider et al., 1972a). Buoyant densities of particles containing the satellite RNA range from 1.408 to 1.529 in increments of 0.009 (Schneider et al., 1972a).
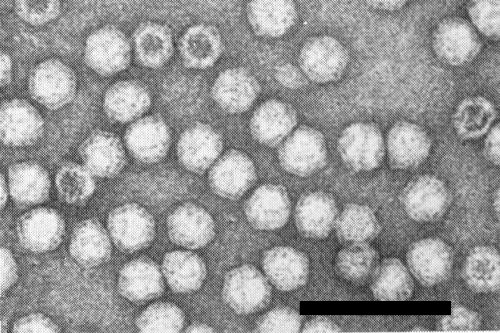
Particle Structure
Particles are c. 28 nm in diameter, with angular outlines, and have 60 structural subunits (Mayo et al., 1971). Electron micrographs show some particles completely, some partially and some not penetrated by phosphotungstate (Fig. 7). Particles penetrated by negative stain do not necessarily represent particles devoid of RNA; the proportion of particles penetrated increases with increasing pH of the stain but the staining time has no effect on penetration (Davison & Francki, 1969).
Particle Composition
Nucleic acid: RNA, single-stranded. The genome comprises two essential RNA molecules, RNA-1 and RNA-2 (Harrison et al., 1972), with M. Wt of 2.73 x 106 and 1.34 x 106 respectively, estimated by electrophoresis of glyoxylated RNA in 0.75% agarose gels (Murant et al., 1981). The sedimentation coefficient of RNA-1 is c. 32 S and that of RNA-2 c. 24 S. For unfractionated RNA, the percentage nucleotide composition is G:A:C:U = 24.7:23.1:22.4:29.8 (Stace-Smith et al., 1965).
Protein: Coat protein can be isolated by disrupting virus in 1.0 M HCl at room temperature for 24 h (Stace-Smith et al., 1965) or by heating for 2 h at 55-60°C in 0.1 M phosphate buffer, pH 8.0 (Chu & Francki, 1979). The protein subunit has a M. Wt of approximately 57,000 (Mayo et al., 1971) although there is evidence to suggest that each subunit is a stable tetramer of a smaller protein with a M. Wt of c. 13,000 (Chu & Francki, 1979).
Genome Properties
Each RNA species contains polyadenylate, apparently at the 3' end (Mayo et al., 1979a) and a protein of M. Wt c. 4000 attached covalently, probably at the 5' end (Mayo et al., 1979b). This genome-linked protein is apparently needed for infectivity because infectivity is abolished by incubation with Pronase or proteinase K (Harrison & Barker, 1978; Mayo et al., 1982) but it is not necessary for viral RNA translation in vitro in a wheat germ system (Chu et al., 1981). In this system and in the rabbit reticulocyte lysate system, RNA-1 is translated into proteins of M. Wt 25,000 and 205,000, one or both of which is processed to produce proteins of M. Wt 195,000 and 135,000; RNA-2 is translated into protein predominantly of M. Wt 116,000 which is processed in reticulocyte lysates by a protein induced by RNA-1 into products of M. Wt 53,000, 40,000 and 23,000. The 53,000 M. Wt product is precipitated by antiserum to tobacco ringspot virus and is presumably the virus coat protein (Forster & Morris-Krsinich, 1985).
Satellite
Small satellite ssRNA molecules which depend upon the genomic RNA molecules of the virus for coat protein and for replication functions have been detected in the particles of some isolates of the virus (Schneider, 1971; Schneider et al., 1972a,1972b; Rezaian, 1980). The most abundant molecule (RNA-S) has a M. Wt of approximately 120,000 (Sogo et al., 1974) but at least 10 covalent multimeric forms of this molecule were detected in particle preparations by electrophoretic analysis (Kiefer et al., 1982). Neither polyadenylate sequences nor covalently linked protein were detected in RNA-S or its more slowly migrating forms, and the biological activity of RNA-S was not protease-sensitive (Kiefer et al., 1982). In addition, plants infected with the virus and RNA-S yielded a multicomponent population of RNA molecules having some of the properties of dsRNA (Schneider & Thompson, 1977). Electron microscopy of such material revealed linear molecules of various lengths but also circular molecules and racket-like structures (Sogo & Schneider, 1982). When denatured, this partially double-stranded RNA fraction gives a series of up to 12 electrophoretic components that contain both RNA-S sequences and sequences that hybridize with RNA-S; they represent an oligomeric series of (+) and (-) stranded satellite RNA sequences (Kiefer et al., 1982). Linthorst & Kaper (1984) found circular single-stranded monomeric and dimeric forms of the satellite in infected tissue but detected only linear (+) stranded monomers and dimers in RNA from purified virus particles. The evidence suggests that RNA-S molecules replicate via a rolling circular mechanism analogous to that proposed for viroid replication (Kiefer et al., 1982; Linthorst & Kaper, 1984).
Relations with Cells and Tissues
The virus particles are morphologically similar to ribosomes and individual particles are consequently difficult to identify in thin sections of infected tissue. However, they can be recognized when they associate in characteristic ways, e.g. within tubules, in crystalline arrays or in unstructured aggregates. Files of particles, enclosed in tubules and frequently associated with plasmodesmata, were detected in the root tips of infected French bean (Crowley et al., 1969), in the meristematic cells of tobacco (Roberts et al., 1970; Walkey & Webb, 1970), and in the cell walls of infected soybean seed (Yang & Hamilton, 1974). Particle aggregates were not detected in root-tip or leaf cells of infected French bean, cucumber or cowpea (Crowley et al., 1969) but they were observed in soybean in the bundle sheath (Halk & McGuire, 1973), and in the intine of the pollen wall, the wall and the cytoplasm of the generative cell, the integuments, nucellus, embryo sac wall and megagametophytic cells (Yang & Hamilton, 1974).
Notes
Tobacco ringspot virus may be confused with several other nepoviruses when identification is based on the symptom response in a range of herbaceous indicator plants. Of the various nepoviruses, tomato ringspot is the one most likely to be confused with tobacco ringspot because these two viruses commonly infect the same species and occur in the same geographical region. McLean (1962) reported that 11 of 43 species that were tested showed differential reactions to the two viruses. Despite the possibility of distinguishing these viruses on the basis of host reactions, serological tests are essential for positive identification.
Figures

Sucrose density gradient profile of a purified preparation showing top (T), middle (M) and bottom (B) components. Gradient was centrifuged in a Beckman SW41 rotor at 4°C, 38 000 rev/min for 90 min.